OVERVIEW
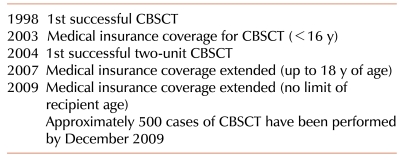
Based on the study result that the cord blood contains hematopoietic stem cells which are capable of regenerating blood cells, the first cord blood stem cell transplantation (CBSCT) was successfully performed at France in 1988. Since the first successful CBSCT was performed in 1998, more than 500 patients were treated with CBSCT in Korea (Table 1) [1]. Afterwards, cord blood was used in a variety of hematologic and oncologic diseases that can be cured by hematopoietic stem cell transplantation, and cord blood banks were beginning to be established for cryopreservation of cord bloods that were otherwise discarded. Along with this movement, active studies on adult stem cell have arisen and the attention was focused on cord blood as an important source for cell therapy [2].
Public cord blood banks began to be established in order to utilize cord bloods that were once discarded, and these banks have been operated by fundings of the university hospitals, public organizations, and sponsorship organizations worldwide. Countries that are most representative in supporting such cord blood banks with national budgets are the United States (US) and Japan. The US government officially recognized cord blood bank project along with bone marrow bank project based on 『Stem Cell Act - C.W. Bill Young Cell Transplantation Program』 in 2005 [3]. They devised an independent budget for new cord blood bank project and secured 60 million dollars of national budget for the preservation of 150,000 units of cord blood. Also, in addition to the operation of hematopoietic stem cell related program that includes cord blood in association with National Marrow Donor Program, the US Health Resources & Services Administration in the Department of Health and Human Services supports budgets to cord blood banks by direct negotiations based on Stem Cell Act 2005. In Japan, more than 3 times the budget for organ transplantation project is being provided for the managements of bone marrow and cord blood. The budget for public cord blood banking project is used, in part, for the laboratory tests and processing of cord bloods for banking as well as networking business of Japan Cord Blood Bank Network via Japan Red Cross [4].
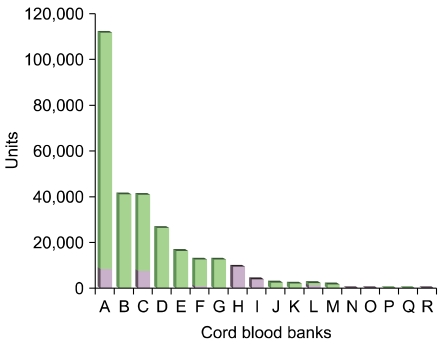
NetCord, a global cord blood network, is being operated to use donated cord blood for public purposes, and more than 200,000 units are currently registered as of February, 2010. In Korea, we established the networking system of 7 public cord blood banks (KoreaCORD) in October 2006 and more than 20,000 units are currently registered as of December 2009. On the other hand, private cord blood banks that centered around the US have opened their business to commercially preserve cord bloods under the agreement with contractor. The need for such business is in big controversy, but millions of units were estimated to have been preserved around the globe already and more than 200,000 units are preserved in Korea (Fig. 1).
Although the cord blood preserved in cord blood banks are abided by international standards, the quality management is partially dependent on internationally recognized non-government organization called the Foundation for the Accreditation of Cellular Therapy, or on self-institutional cord blood bank standards. These cord bloods are known to have no big differences in terms of hematopoietic potentials even after 15 years of cryopreservation, but since long-term cryopreservation of cord blood is known to yield substantial number of cell death according to some studies, questions have been raised for the effect of long-term preservation. These preserved cord bloods are mostly for the purpose of hematopoietic stem cell transplantation. However, since many positive study results on cell therapy are being reported recent days, utilization of cord bloods as resources for cell therapy would also be necessary.
In order for wide clinical application of cord bloods, points such as donor searching system, and funds for expansion of donated cord blood pools, establishing quality management system of preserved cord bloods, and utilizing the cord blood bank as resources for various cell therapies need to be considered. Therefore, national level of the management system should be launched and basic researches for the quality control and utilization of cryopreserved cord bloods should be implemented. For these reasons, the 『Cord Blood Management and Research Act』 was approved by the Korea National Assembly in February 2010. According to this Act, Cord Blood Committee will be constituted under the Ministry of Health and Welfare for strict quality management of cord blood, and it will be required to deliberate on things like setting cord blood management policies and eligibility standards of cord blood donors, as well as the approval of cord blood bank, and etc. The Act also allows the complete or partial funding for public cord blood bank establishment and operation within the possible budget range when the national or regional government plans for establishment or assignment of public cord blood bank. In addition, it states the operation of Cord Blood Coordinating Center for cord blood search and management [5]. In order for cord blood management project to be effective based on these legal evidences, the cord blood management project should create quality management basis by broad research projects on such topic as the safety and efficacy of long-term cryopreserved cord blood along with an amendment of『Cord Blood Bank Standard Operating Procedure』 officially announced in August 2005. Furthermore, the cord blood bank should not only be utilized for the purpose of hematopoietic cell transplantation but also for cell therapy via funding of basic research, which would then lead to the creation of high-value bioresource along with great benefit to national economics.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download