Abstract
Background
On performing umbilical cord blood (UCB) transplantation, faster engraftment may lead better clinical outcome. Because transplanted viable cell count in UCB is related to the engraftment, accurate evaluation of viability of CD34+ cells in cryopreserved UCB has clinical implication. We examined the difference in viability of cells in cryopreserved UCB according to the duration of cryopreservation and different methods.
Methods
A total of 60 UCB samples which were cryopreserved for 1 to 4 years were used in this study. Viability of cryopreserved cells were examined with trypan blue exclusion assay, DNA contents analysis, caspase-3 activation test, intracellular esterase activity and Annexin-V/PI staining.
Results
After thawing the cryopreserved UCB, 89% of the total MNCs and 84% of CD34+ cells were viable as identified by trypan blue exclusion assay. In the CD34+ cell population, the cell death rate was found to be 47% by Annexin-V/PI staining and less than 5% by DNA contents analysis. However, cspase-3 activity failed to document apoptosis. The intracellular esterase activity test also showed a cell death rate of about 10∼20% at 2, 4, and 6 hours after thawing.
Conclusion
Viable cells in UCB should be measured by several compensatory techniques rather than a single method. Discordance among Annexin-V/PI staining versus trypan blue exclusion, DNA contents analysis, and the caspase-3 activation test or intracellular esterase activity should be clarified in order to apply these techniques for actual cord blood transplantation.
References
1. Rocha V, Wagner JE Jr, Sobocinski KA, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an hla-identical sibling. Eurocord and international bone marrow transplant registry working committee on alternative donor and stem cell sources. N Engl J Med. 2000; 342:1846–54.
2. Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998; 339:1565–77.

3. Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989; 86:3828–32.

4. Broxmeyer HE, Kurtzberg J, Gluckman E, et al. Umbilical cord blood hematopoietic stem and repopulating cells in human clinical transplantation. Blood Cells. 1991; 17:313–29.
5. Gluckman E, Broxmeyer HA, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fan-coni's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989; 321:1174–78.

6. Kamani N, Spellman S, Hurley CK, et al. State of the art review: HLA matching and outcome of unrelated donor umbilical cord blood transplants. Biol Blood Marrow Transplant. 2008; 14:1–6.

7. Lister J, Gryn JF, McQueen KL, Harris DT, Rossetti JM, Shadduck RK. Multiple unit HLA-unmatched sex-mismatched umbilical cord blood transplantation for advanced hematological malignancy. Stem Cells Dev. 2007; 16:177–86.

8. Wagner JE, Rosenthal J, Sweetman R, et al. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host disease. Blood. 1996; 88:795–802.

9. Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002; 100:1611–8.

10. Shim JS, Cho B, Kim M, et al. Early apoptosis in CD34+ cells as a potential heterogeneity in quality of cryopreserved umbilical cord blood. Br J Haematol. 2006; 135:210–3.
11. Theunissen K, Verfaillie CM. A multifactorial analysis of umbilical cord blood, adult bone marrow and mobilized peripheral blood progenitors using the improved ML-IC assay. Exp Hematol. 2005; 33:165–72.

12. Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980; 68:251–306.

13. Mesner PW Jr, Kaufmann SH. Methods utilized in the study of apoptosis. Adv Pharmacol. 1997; 41:57–87.

14. de Boer F, Dräger AM, Pinedo HM, et al. Early apoptosis largely accounts for functional impairment of CD34+ cells in frozen-thawed stem cell grafts. J Hematother Stem Cell Res. 2002; 11:951–63.
15. Mastino A, Favalli C, Camilli AR, Malerba C, Grelli S, Caluqi A. Umbilical cord blood: the role of apoptosis in the control of CD34+ cell counts. Placenta. 2003; 24:113–5.

16. Schuurhuis GJ, Muijen MM, Oberink JW, de Boer F, Ossenkoppele GJ, Broxterman HJ. Large populations of non-clonogenic early apoptotic CD34-positive cells are present in frozen-thawed peripheral blood stem cell transplants. Bone Marrow Transplant. 2001; 27:487–98.

17. Glander HJ, Schaller J. Binding of annexin v to plasma membranes of human spermatozoa: a rapid assay for detection of membrane changes after cryosto-rage. Mol Hum Reprod. 1999; 5:109–15.

18. Balasubramanian K, Mirnikjoo B, Schroit AJ. Regulated externalization of phosphatidylserine at the cell surface: implications for apoptosis. J Biol Chem. 2007; 282:18357–64.
19. Duru NK, Morshedi M, Schuffner A, Oehninger S. Cryopreservation-thawing of fractionated human spermatozoa and plasma membrane translocation of phosphatidylserine. Fertil Steril. 2001; 75:263–8.

20. Duru NK, Morshedi MS, Schuffner A, Oehninger S. Cryopreservation-thawing of fractionated human spermatozoa is associated with membrane phosphatidylserine externalization and not DNA fragmentation. J Androl. 2001; 22:646–51.
21. Muller K, Pomorski T, Muller P, Herrmann A. Stability of transbilayer phospholipid asymmetry in viable ram sperm cells after cryotreatment. J Cell Sci. 1999; 112:11–20.

Fig. 1.
Mononuclear cell (MNC) viabilities of cryopreserved umbilical cord blood (UCB) samples after thawing. The mean and standard deviation value of viable cell percents from 60 umbilical cord blood (UCB) samples was 88.9±6.8 by trypan blue staining (A). The result was not affected by the duration of cryopreservation (B).
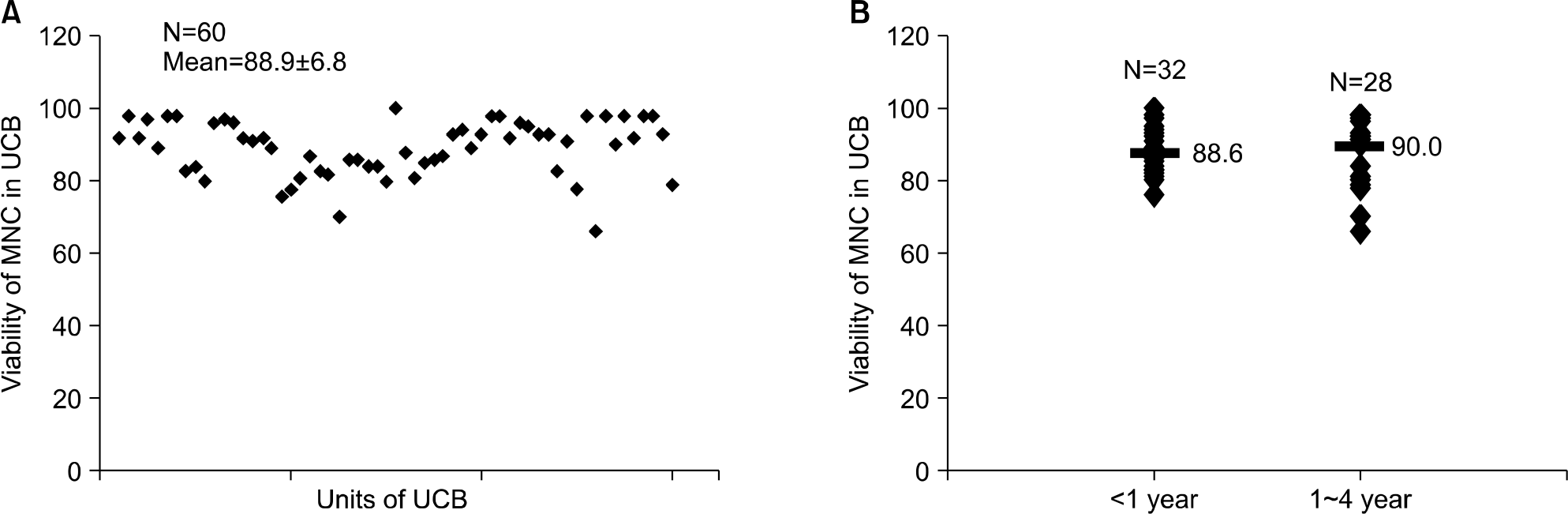
Fig. 2.
Successfully isolated CD34+ cells (purity of 83%) showed 83.9% of viability similar to MNC viability (A, B). Live cells and dead cells were detected by esterase activity and EthD-1, respectively (C).
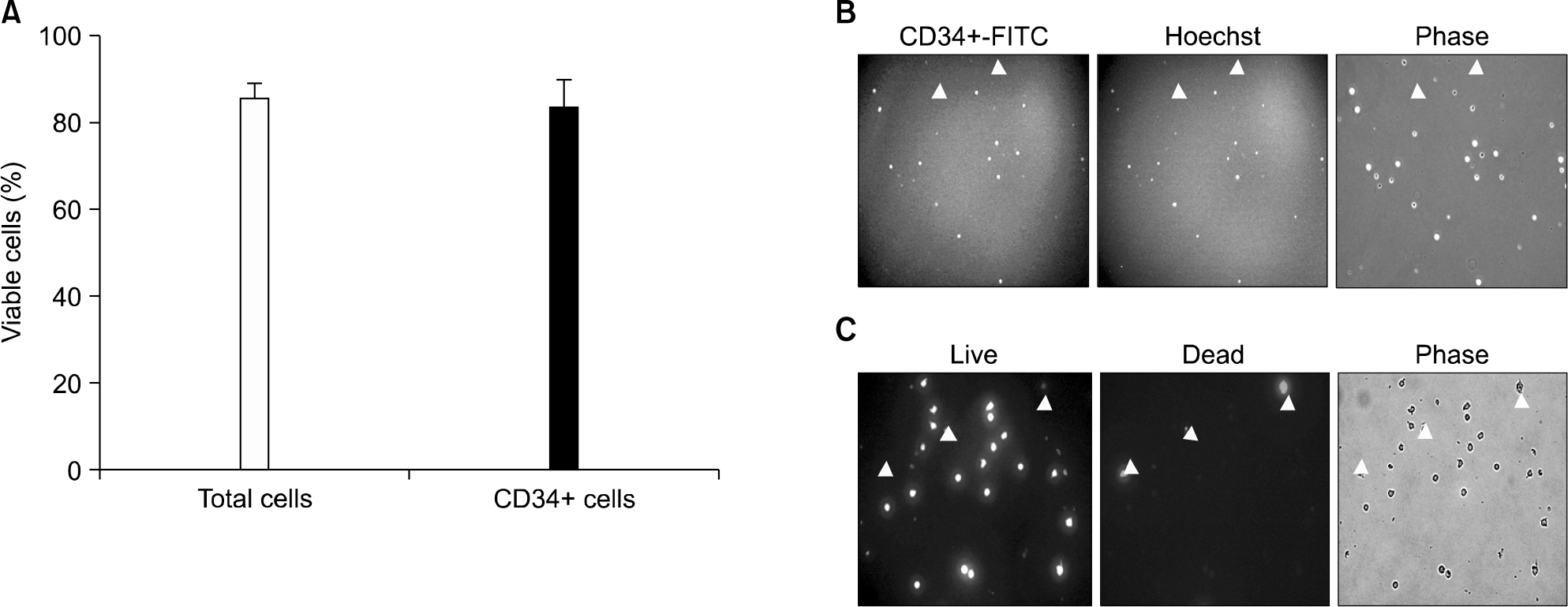
Fig. 3.
33.1±9.9% and 16.5±9.3% of CD34+ cells expressed Annexin-V/PI (+/?) (early apoptosis) and Annexin-V/PI (+/+) cells (late apoptosis), respectively (A). However DNA contents analysis showed only 3.1±1.2% of CD34+ cells lost their 2N meaning apoptosis (B, C). Profound discordance was observed between two techniques, Annexin-V/PI staining and DNA contents analysis. 5 out of 10 units UCB selected randomly were represented.
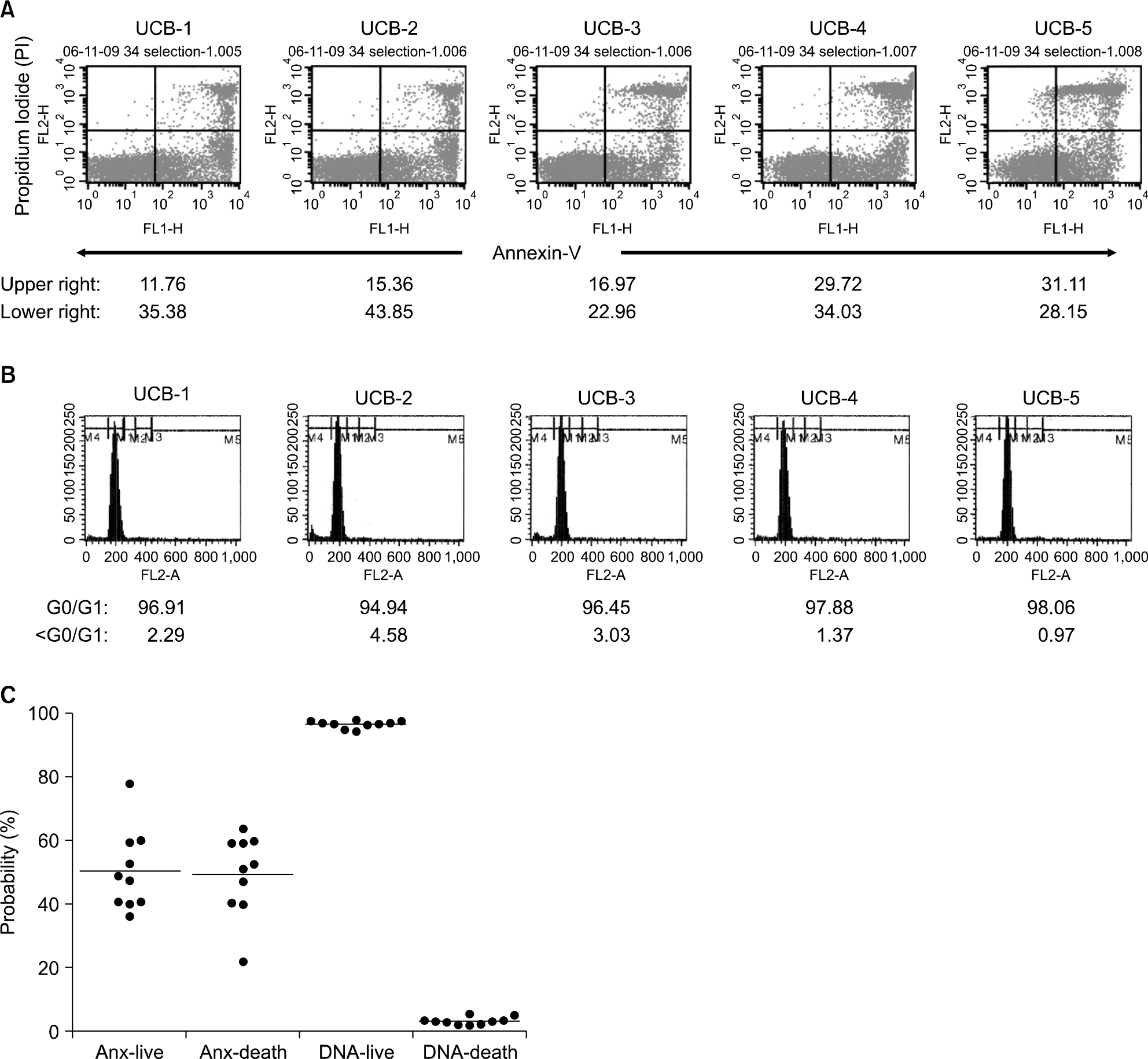
Fig. 4.
Caspase-3 activity test for CD34+ cells from randomly selected 10 samples. UCB #3 sample showed faint caspase-3 activity compared with apop-tosis-induced tumor cell line (A). CD34+ UCB cells did not express active or cleaved form of caspase-3 regardless of Annexin-V or Propidium Iodide (PI) positivity (B). Actual activity of caspase-3 should be examined by cleaved fraction which was not detected at any sample (C). T, tumor cell line (neuroblastoma); T+A, apoptosis-induced tumor cell line.
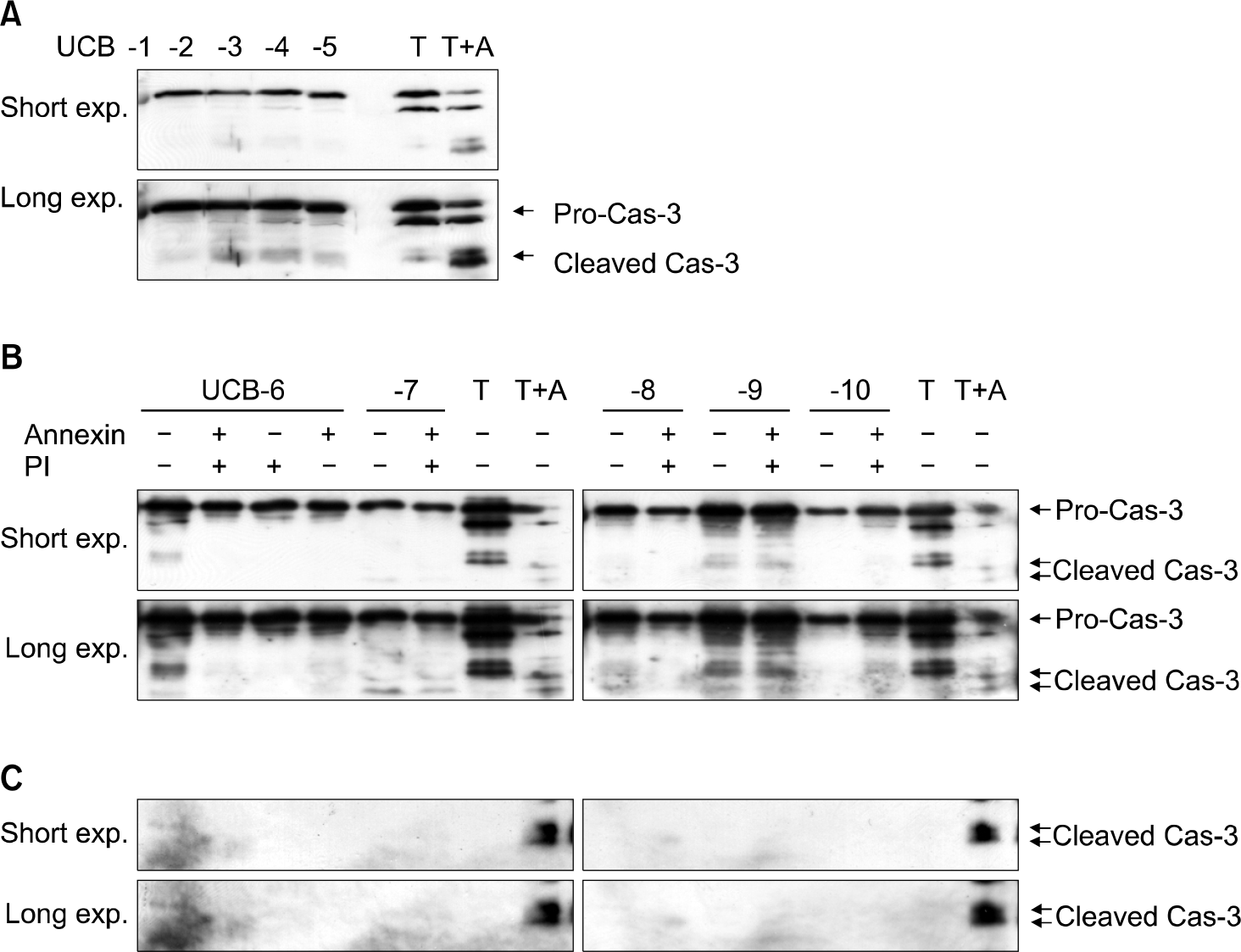
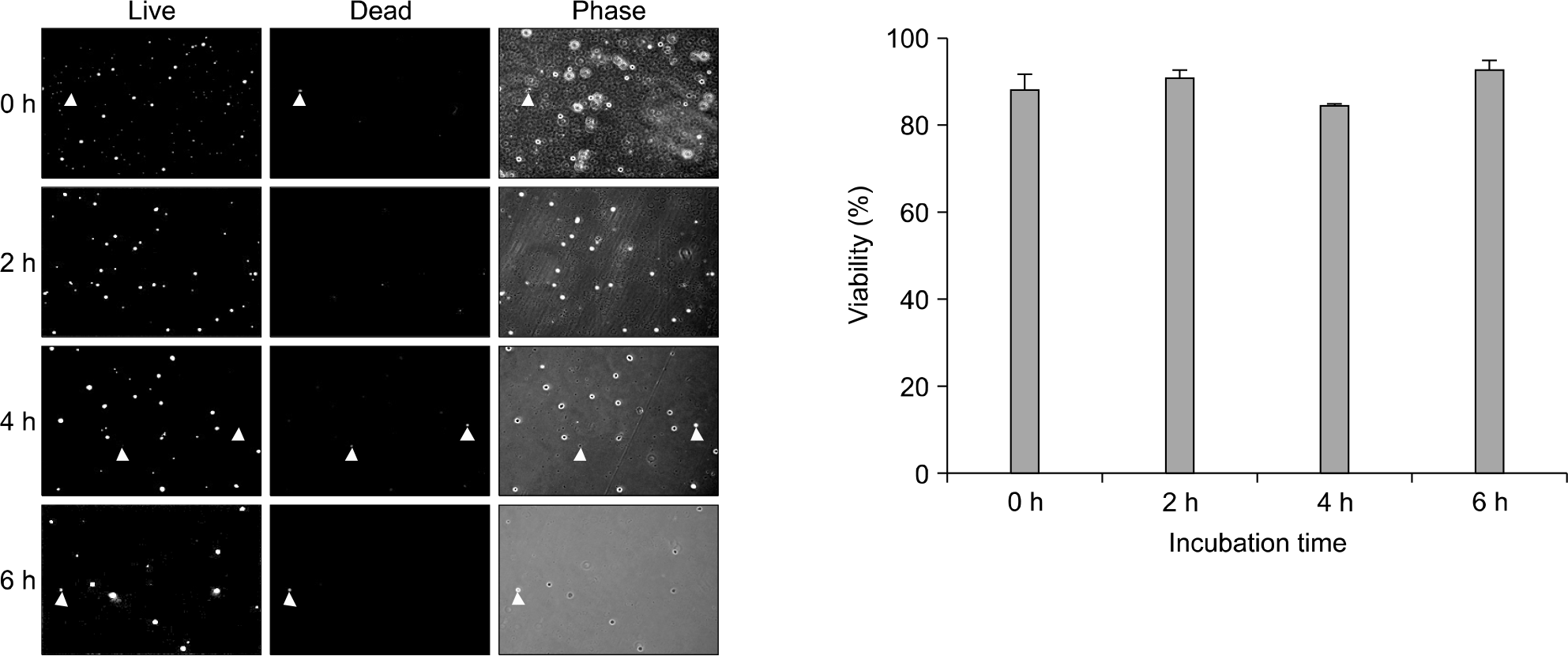
Fig. 5.
Result of intracellular esterase activity test for CD34+ cells. Randomly selected one sample was checked its intracellular esterase activity at 0, 2, 4, 6 hours after thawing. The cells were incubated in RPMI1,640 media at CO2 incubator. There was no decrement of esterase activity which means CD34+ UCB cells do not die at least within 6 hours.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download