Abstract
Background
Acute leukemias co-expressing myeloid and lymphoid antigens but does not meet the criteria for biphenotypic acute leukemia (BAL) is common, however its clinical significance is not fully defined.
Methods
In this study, clinical features of 68 co-expressing (myeloid and lymphoid) acute leukemias diagnosed between January 2000 and December 2006 were studied and compared with those of a control group of patients (pure AML or ALL).
Results
Age, gender, initial Lactate dehydrogenase (LDH) level and cytogenetics were not different between the co-expressing group and the control group. But, the initial bone marrow blast percent was significantly higher in the co-expressing group (70% vs. 54.5%, P=0.003). Fifty five percent (16/29) of ALL and 30% (52/172) of AML patients showed myeloid and lymphoid markers concomitantly. The lymphoid antigen positive AML (Ly+ AML) patients showed significantly shorter survival rates than pure AML patients (4 year survival rate, 17.6% vs. 45.6%, P=0.002). However hematopoietic stem cell transplantation (HST) abrogated the difference (4 year survival rate, 54.7% vs. 50.6%, P=0.894). In ALL patients, survival rate was not affected by myeloid antigen co-expression (4 year survival rate 26.1% vs. 20%, P=0.954).
Go to : 
References
1. Khalidi HS, Medeiros LJ, Chang KL, Brynes RK, Slovak ML, Arber DA. The immunophenotype of adult acute myeloid leukemia: high frequency of lymphoid antigen expression and comparison of immunophenotype, french-american-british classification, and karyotypic abnormalities. Am J Clin Pathol. 1998; 109:211–20.
2. Launder TM, Bray RA, Stempora L, Chenggis ML, Farhi DC. Lymphoid-associated antigen expression by acute myeloid leukemia. Am J Clin Pathol. 1996; 106:185–91.

3. Kawai S, Zha Z, Yamamoto Y, Shimizu H, Fujimoto T. Clinical significance of childhood acute myeloid leukemias expressing lymphoid-associated antigens. Pediatr Hematol Oncol. 1995; 12:463–9.

4. Matutes E, Morilla R, Farahat N, et al. Definition of acute biphenotypic leukemia. Haematologica. 1997; 82:64–6.
5. Legrand O, Perrot JY, Simonin G, et al. Adult biphenotypic acute leukaemia: an entity with poor prognosis which is related to unfavourable cytogenetics and P-glycoprotein over-expression. Br J Haematol. 1998; 100:147–55.

6. Killick S, Matutes E, Powles RL, et al. Outcome of biphenotypic acute leukemia. Haematologica. 1999; 84:699–706.
7. Del Poeta G, Stasi R, Venditti A, et al. CD7 expression in acute myeloid leukemia. Leuk Lymphoma. 1995; 17:111–9.
8. Putti MC, Rondelli R, Cocito MG, et al. Expression of myeloid markers lacks prognostic impact in children treated for acute lymphoblastic leukemia: Italian experience in AIEOP-ALL 88–91 studies. Blood. 1998; 92:795–801.

9. Ball ED, Davis RB, Griffin JD, et al. Prognostic value of lymphocyte surface markers in acute myeloid leukemia. Blood. 1991; 77:2242–50.
10. Kantarjian HM, O'Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000; 18:547–61.

11. Wiernik PH, Banks PL, Case DC Jr, et al. Cytarabine plus idarubicin or daunorubicin as induction and consolidation therapy for previously untreated adult patients with acute myeloid leukemia. Blood. 1992; 15:313–9.

12. Bene MC, Bernier M, Castoldi G, et al. Impact of immunophenotyping on management of acute leukemias. Haematologica. 1999; 84:1024–34.
13. Uckun FM, Sather HN, Gaynon PS, et al. Clinical features and treatment outcome of children with myeloid antigen positive acute lymphoblastic leukemia: a report from the children's cancer group. Blood. 1997; 90:28–35.
14. Childs CC, Hirsch-Ginsberg C, Walters RS, et al. Myeloid surface antigen-positive acute lymphoblastic leukemia (My+ ALL): immunophenotypic, ultrastructural, cytogenetic, and molecular characteristics. Leukemia. 1989; 3:777–83.
15. Wiersma SR, Ortega J, Sobel E, Weinberg KI. Clinical importance of myeloid-antigen expression in acute lymphoblastic leukemia of childhood. N Engl J Med. 1991; 324:800–8.

17. Baruchel A, Cayuela JM, Ballerini P, et al. The majority of myeloid-antigen-positive (My+) childhood b-cell precursor acute lymphoblastic leukaemias express tel-aml1 fusion transcripts. Br J Haematol. 1997; 99:101–6.
Go to : 
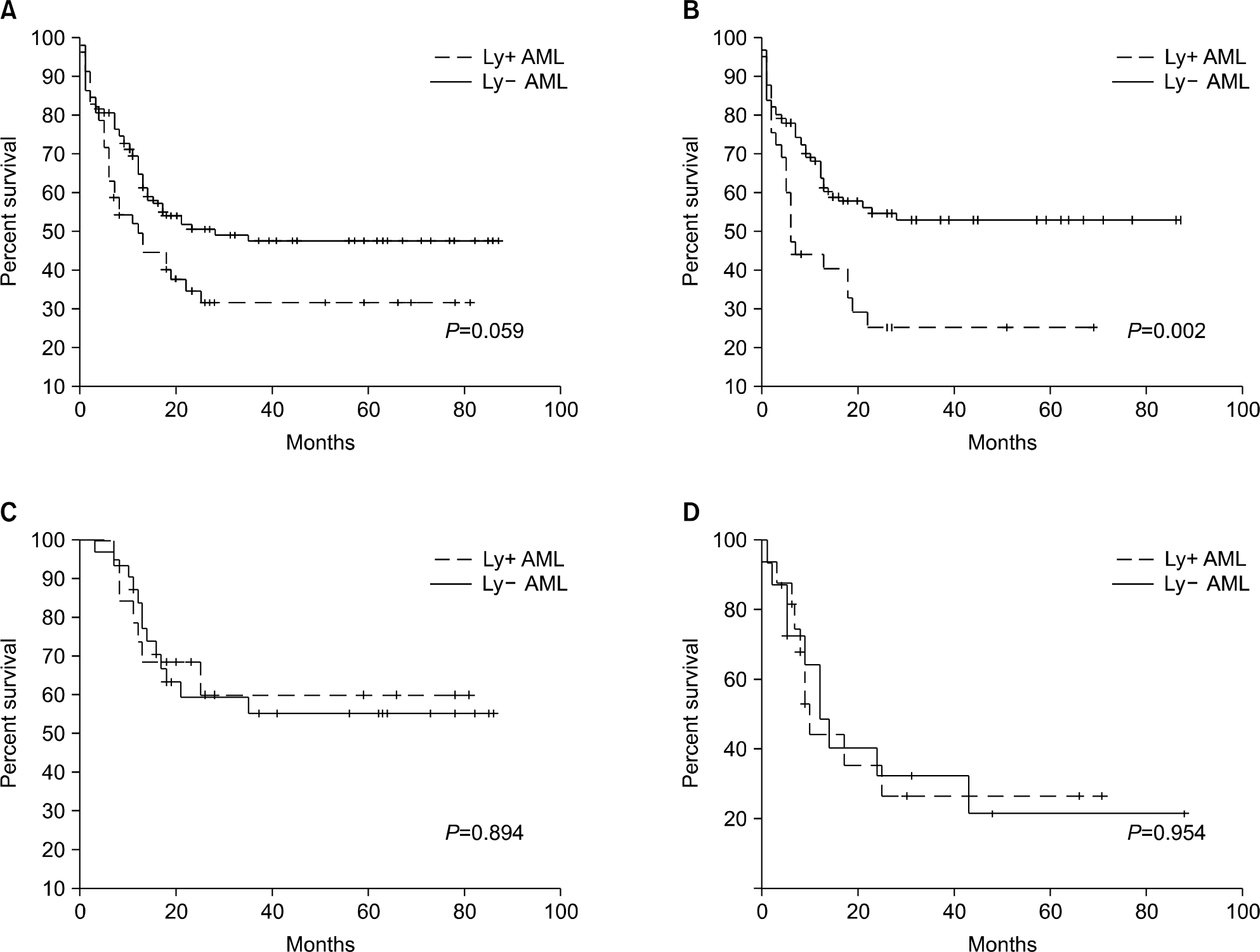 | Fig. 1.Survival of patients with co-expression against matched control. (A) Overall survival of AML patients, (B) Survival of AML patients without HST. (C) Survival of AML patients with HST. (D) Overall survival of ALL patients. |
Table 1.
Panel of antibodies used
| B-lymphoid | T-lympho | id Myeloid | Non-lineage Ag |
|---|---|---|---|
| CD10 | CD2 | CD13 | CD34 |
| CD19 | CD3 | CD14 | HLA-DR |
| CD20 | CD5 | CD33 | |
| CD22 | CD7 | anti-MPO∗ | |
| CD79a | Tdt† |
Table 2.
Clinical characteristics of the patients
Table 3.
Co-expression profiles of acute leukemia
Table 4.
Details of treatment in AML
Abbreviations: Ly? AML, lymphoid antigen positive AML; Ly+ AML, lymphoid antigen negative AML; HST, hematopoietic stem cell transplantation; Auto-PBSCT, autologous peripheral blood stem cell transplantation; Allo-BMT, allogeneic bone marrow transplantation; Allo-PBSCT, allogeneic peripheral blood stem cell transplantation; TRM, treatment related mortality.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download