Abstract
Congenital immunodeficiency is one or combined immune defect in immunoglobulin, leukocyte, and complement. These patients have increased susceptibility to respiratory infection. Hence, their infection must be taken care of, tried to gene therapy and stem cell transplantation. We present here a case of hyper-IgM syndrome in an 11-year-old male patient who complained of abdominal distension and abdominal pain. Multiple abdominal masses were detected by abdominal computed tomography (CT) and he was diagnosed with neuroendocrine carcinoma by mass biopsy. There was no evidence of metastasis of cancer cells to the bone marrow, but a dysgranulopoietic feature was noted and he was diagnosed with childhood myelodysplastic syndrome. This is the first report that neuroendocrine carcinoma is associated with childhood myelodysplastic syndrome in hyper-IgM syndrome.
References
1. Carneiro-Sampaio M, Coutinho A. Immunity to microbes: lessons from primary immunodeficiencies. Infect Immun. 2007; 75:1545–55.

2. Hahn YS. Detection and diagnosis of primary immunodeficiency diseases. Korea J Pediat. 2004; 47:475–9.
3. Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol. 1999; 93:190–7.
4. Lim MS, Elentitoba-Johnson KS. The molecular pathology of primary immunodeficiencies. J Mol Diagn. 2004; 2:59–83.

5. Lee WI, Torgerson TR, Schumacher MJ, Yel L, Zhu Q, Ochs HD. Molecular analysis of a large cohort of patients with the hyper immunoglobulin M (IgM) syndrome. Blood. 2005; 105:1881–90.

6. Puck JM, Malech HL. Gene therapy for immune disorders: good news tempered by bad news. J Allergy Clin Immunol. 2006; 117:865–9.

7. Al-Ghonaium A. Stem cell transplantation for primary immunodeficiencies: king faisal specialist hospital experience from 1993 to 2006. Bone Marrow Transplant. 2008; 42:S53–6.

8. Lougaris V, Badolato R, Ferrari S, Plebani A. Hyper immunoglobulin M syndrome due to CD40 deficiency: clinical, molecular, and immunological features. Immunol Rev. 2005; 203:48–66.

9. Knowles DM. Immunodeficiency-associated lymphoproliferative disorders. Mod Pathol. 1999; 12:200–17.
10. Revy P, Muto T, Levy Y, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the hyper-IgM syndrome (HIGM2). Cell. 2000; 102:565–75.

11. Hayward AR, Levy J, Facchetti F, et al. Cholan-giopathy and tumors of the pancreas, liver, and Biliary tree in boys with X-linked immunodeficiency with hyper-IgM. J Immunol. 1997; 158:977–83.
12. Erdos M, Garami M, Rákóczi E, et al. Neuroendocrine carcinoma associated with X-linked hyperimmunoglobulin M syndrome: report of four cases and review of the literature. Clin Immunol. 2008; 129:455–61.
13. Malhotra RK, Li W. Poorly differentiated gastroenteropancreatic neuroendocrine carcinoma associated with X-linked hyperimmunoglobulin M syndrome. Arch Pathol Lab Med. 2008; 132:847–50.

Fig. 1.
Abdominal CT scan findings. (A) Horizontal abdominal CT scan revealed an 85×79 mm cystic change of tumor mass in the transverse colon, multiple enlarged mass around colon, and in the liver. (B) Transversal abdominal CT scan showed multiple variable sized liver lesions.
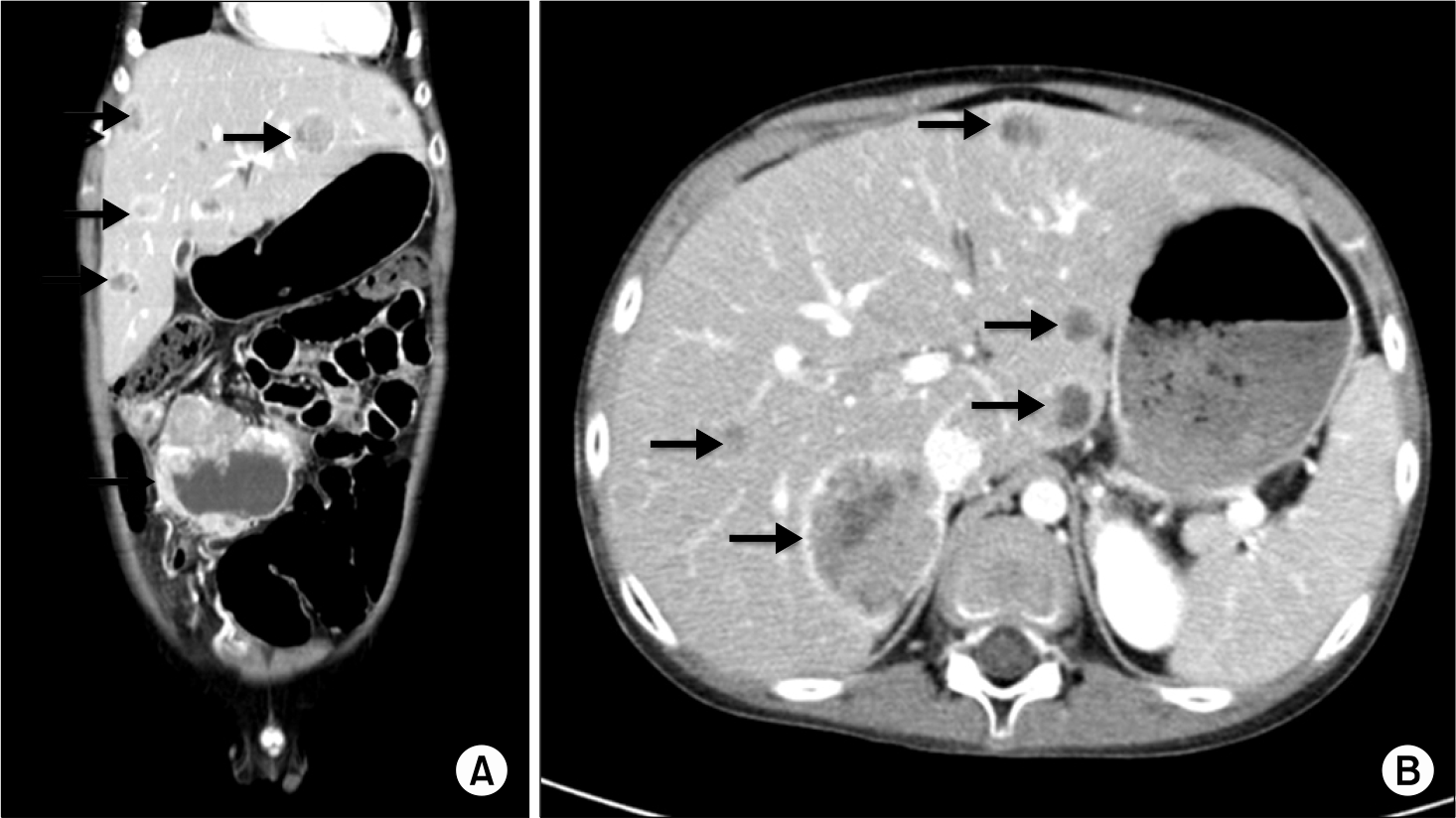
Fig. 2.
Microscopic finding of the abdominal mass. (A) Compact tumor cells and sheaths proliferations consistent with small round cell cancer (H&E stain, ×200). (B) Immunohistochemically tumor cells show strong positivity for the synaptophysin (synaptophysin stain, x200).
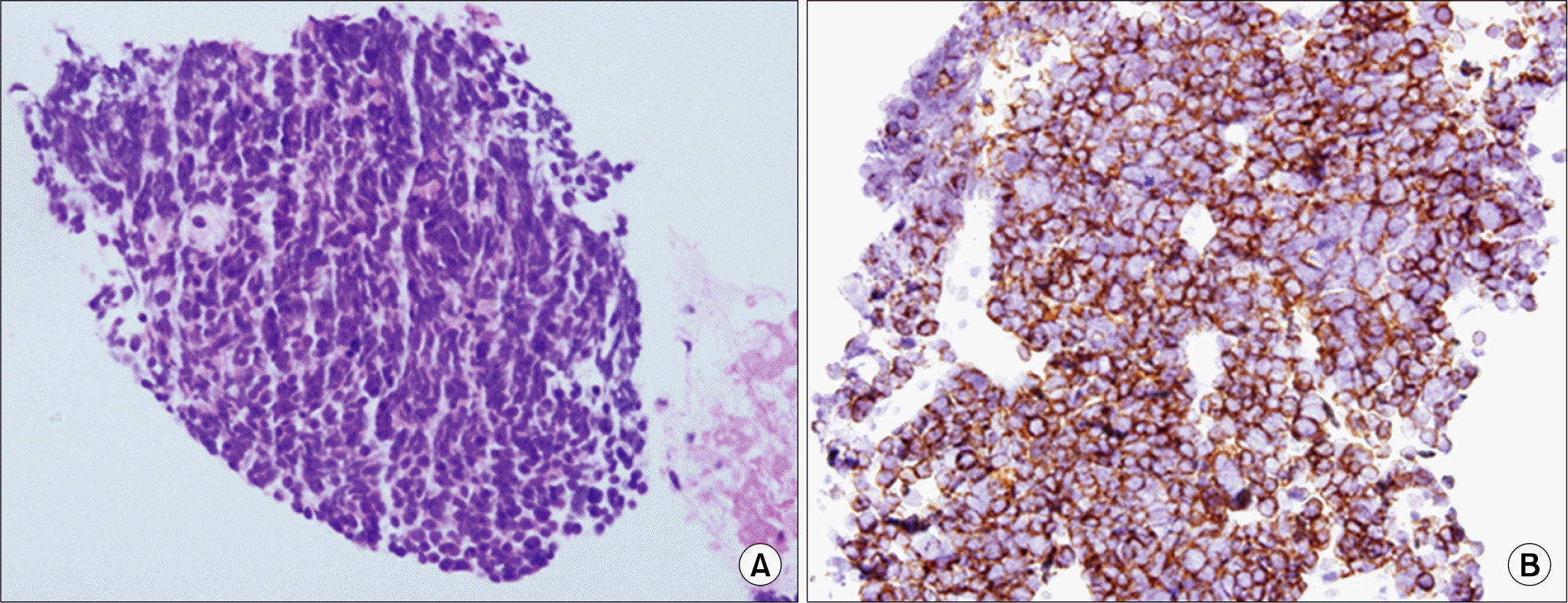
Fig. 3.
Microscopic finding of peripheral blood and bone marrow aspiration. (A) Neutrophil showed megaloblastic change (PB, Wright stain, ×1,000). (B) Megaloblastic change and abnormal lobulated neutrophils (BM, Wright stain, ×1,000).
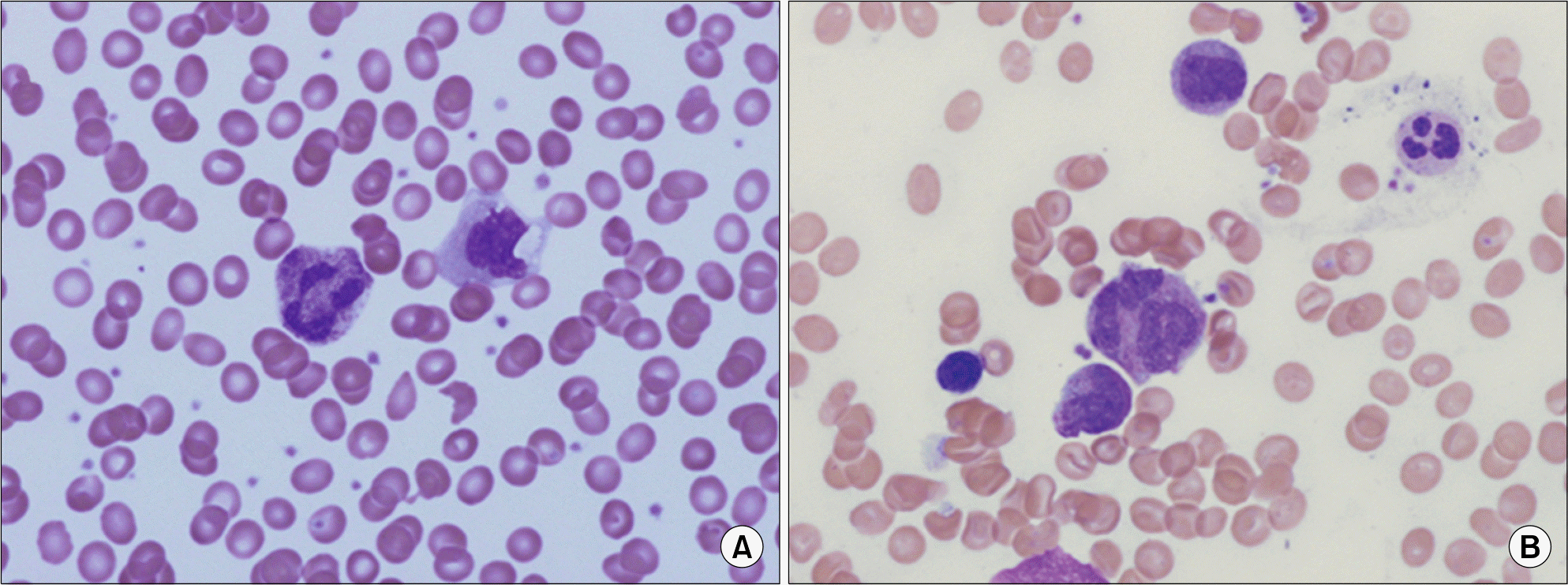
Table 1.
The level of serum immunoglobulin and leukocyte from year 2002 to year 2009
| Date | Age (yr-mth) | IgM (g/L) | IgG (g/L) | IgA (g/L) | IgE (IU/mL) | WBC (×109/L) | Neutrophil (×109/L) | Lymphocyte (×109/L) |
|---|---|---|---|---|---|---|---|---|
| 02–6–1 | 4–2 | 2.49 | <2.12 | 0.64 | <0.1 | 0.5 | ||
| 02–8–5 | 4–4 | 1.81 | 3.22 | 0.43 | NT | 4.5 | 1.0 | 3.4 |
| 03–8–4 | 5–4 | 2.95 | 4.32 | 1.11 | <0.1 | 4.9 | 1.1 | 3.1 |
| 04–6–2 | 6–2 | 3.26 | 3.63 | 1.34 | <0.1 | 5.34 | 0.98 | 3.21 |
| 05–7–21 | 7–3 | 3.53 | 3.95 | 1.44 | <0.1 | 4.69 | 0.77 | 3.19 |
| 06–4–12∗ | 8–0 | 3.76 | 3.06 | 0.13 | <0.1 | NT | NT | NT |
| 06–6–12 | 8–2 | 3.74 | 3.61 | 1.80 | <0.1 | 4.26 | 0.49 | 2.82 |
| 07–7–12 | 9–3 | 3.68 | 5.05 | 1.20 | <0.1 | 3.35 | 0.38 | 2.24 |
| 09–1–23† | 11–7 | 1.81 | 3.50 | 0.86 | <0.1 | 4.49 | 2.56 | 1.01 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download