Abstract
Background
Corticosteroids and intravenous immunoglobulin (IV-Ig) have been used asfirst line treatments for acute idiopathic thrombocytopenic purpura (AITP) in children. High dose dexamethasone (HD) has been reported to be effective for chronic refractory ITP and for the initial treatment of AITP in adults. There has been no report about HD as the initial treatment for childhood AITP. We assessed the effectiveness of HD for the initial treatment of childhood AITP, as compared to IV-Ig.
Methods
25 Patients with newly diagnosed AITP were enrolled. We conducted a prospective, randomized study to compare the two treatment options. 11 patients were treated with IV-Ig and 14 patients were treated with HD. The platelet counts were assessed at 3, 5, 7, 14 and 21 days after the beginning of the treatment. The adverse effects were noted, and the patients were followed for more than 6 months.
Results
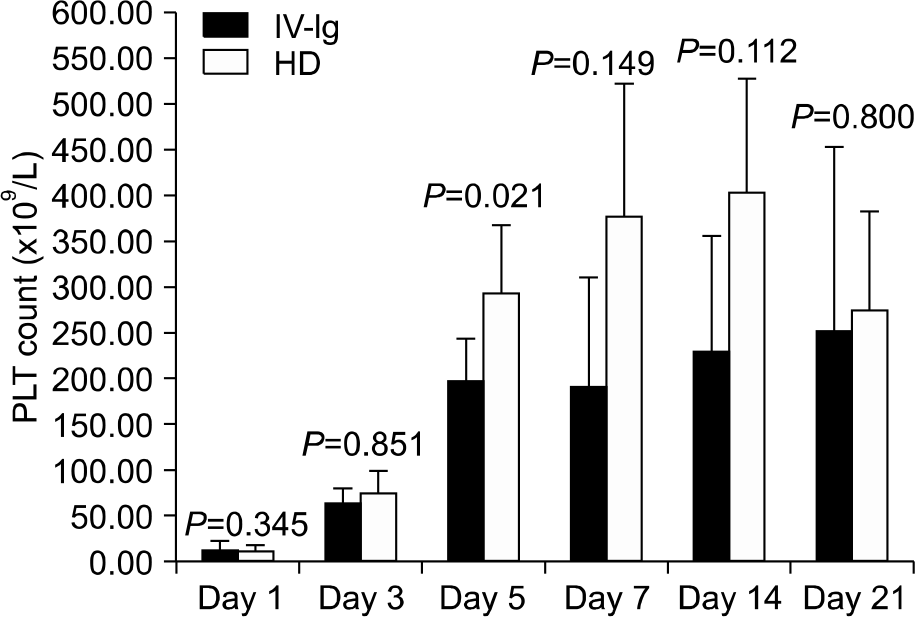
Both the IV-Ig and HD groups showed a rapid rise of the platelet counts and the platelet counts were maintained at 3, 5, 7, 14 and 21 days. The difference of platelet counts between the two groups was significant at day 5 (P<0.05). During the follow-up period, 5 patients had a recurrence: 2 in IV-Ig group and 3 in HD group. All 5 patients were re-treated with HD and they had a good response. One of the recurred patients in the IV-Ig group had chronic ITP. Some side effects were observed, but they were not severe enough to necessitate the discontinuation of treatment.
Go to : 
References
1. Woerner SJ, Abildgaard CF, French BN. Intracranial hemorrhage in children with idiopathic thrombocytopenic purpura. Pediatrics. 1981; 67:453–60.

2. Lilleyman JS. Intracranial hemorrhage hemorrhage in idiopathic thrombocytopenic purpura. Paediatric haematology forum of the british society for haematology. Arch Dis Child. 1994; 71:251–3.
3. Portielje JE, Westendorp RG, Kluin-Nelemans HC, Brand A. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood. 2001; 97:2549–54.

4. Kattamis AC, Shankar S, Cohen AR. Neurologic complications of treatment of childhood acute immune thrombocytopenic purpura with intravenously administered immunoglobulin G. J Pediatr. 1997; 130:281–3.

5. Kim HM. Treatment of immune thrombocytopenic purpura in childhood. Korean J Pediatr. 2004; 47:1262–5.
6. Shin HY. Immune thrombocytopenic purpura (ITP). Korean J Pediatr. 2006; 49:830–2.
7. Kuhne T, Imbach P. Management of children with acute and chronic immune thrombocytopenic purpura. Transfus Sci. 1998; 19:261–8.
8. Borst F, Keuning JJ, van Hulsteijn H, Sinnige H, Vreugdenhil G. High-dose dexamethasone as a first-and second-line treatment of idiopathic thrombocytopenic. purpura in adults. Ann Hematol. 2004; 83:764–8.
9. Cheng Y, Raymond SM, Wong MB. Initial treatment of immune thrombocytopenic purpura with High-dose Dexamethasone. N Engl J Med. 2003; 349:831–6.

10. You CW. The effect of high dose dexamethasone in childhood acute idiopathic immune thrombocytopenia. Korean J Pediatr Hematol-Oncol. 2000; 7:194–202.
11. Blanchette VS, Luke B, Andrew M, et. al. A prospective, randomized trial of high-dose intravenous immune globulin G therapy, oral prednisone therapy, and no therapy in childhood acute immune thrombocytopenia purpura. J Pediatr. 1993; 123:989–95.
12. Mizutani H, Furubayashi T, Imai Y, et al. Mechanisms of corticosteroid action in immune thrombocytopenic purpura (ITP). Experimental studies using ITP-prone mice, (NZWxBXSB) F1. Blood. 1992; 79:942–7.
13. Blanchette VS, Imbach P, Andrew M, et al. Randomised trial of intravenous immunoglobulin G, intravenous anti-D, and oral prednisone in childhood acute immune thrombocytopenic purpura. Lancet. 1994; 344:703–7.

14. Bellucci S, Charpak Y, Chastang C, Tobelem G. Low doses v conventional doses of corticoids in immune thrombocytopenic purpura (ITP): results of a randomized clinical trial in 160 children, 223 adults. Blood. 1998; 71:1165–9.

15. WarrierI. Bussel JB, Valdez L, Barbosa J, Beardsley DS. Safety and efficacy of low dose intravenous immune globulin (IVIG) treatment for Infants and children with immune thrombocytopenic purpura. Low-Dose IVIG study group. J Pediatr Hematol-Oncol. 1997; 19:197–201.
16. Jang JH, Lee MS, Park M, Choeh KC. Comparison of effectiveness between two different protocols of treatment of IV r-globulin in idiopathic thrombocytopenic purpura. Korean J Pediatr. 2003; 46:358–62.
17. Kühne T, Elinder G, Blanchette VS, Garvey B. Current management issues of child and adult immune thrombocytopenic purpura (ITP). Acta Paediatr. 1998; 424:75–81.
18. British Committee for standards in haematology, general haematology task force, working party. Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. British J Haematol. 2003; 120:574–96.
19. Park HY, Kang CG, ShinMY , An KM, Sung KW, Koo HH. Treatment of childhood idiopathic thrombocytopenic purpura with anti-D. Korean J Pediatr Hematol-Oncol. 2000; 7:187–93.
20. Carcao MD, Zipursky A, Butchart S, Leaker M, Blanchette VS. Short course oral prednisone therapy in children presenting with acute immune thrombocytopenic purpura (ITP). Acta Pediatrica. 1998; 424:71–4.
Go to : 
Table 1.
Clinical characteristics of study groups
| HD | IV-Ig | Total | |
|---|---|---|---|
| Cases, n | 14 | 11 | 25 |
| Sex (F/M) | 6/8 | 6/5 | 12/13 |
| Age, months | 38.9±46.1 | 47.5±40.7 | 43.9±42.9 |
| (Mean±SD) |
Table 2.
Platelet response to treatment
Table 3.
Time to reach a platelet count of >20×109/L, > 50×109/L, >100×109/L
| HD | IV-Ig | P-value | |
|---|---|---|---|
| >20×109/L (days) | 2.6±0.5 | 2.6±0.5 | 1.000 |
| >50×109/L (days) | 3.2±1.0 | 3.0±0.0 | 1.000 |
| >100×109/L (days) | 4.6±1.5 | 5.2±0.7 | 0.351 |
Table 4.
Adverse effects of HD and IV-Ig treatment




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download