Abstract
Hematopoietic stem cell transplantation (HSCT) recipients have a risk of post-transplant lymphoproliferative disorder (PTLD), which normally develops in Epstein-Barr virus (EBV) transformed donor B lymphocytes. The incidence of Hodgkin's lymphoma (HL) ranges from 1.8% to 3.4% of PTLD after HSCT. There are no case reports of early onset HL-like PTLD that developed less than one year after HSCT. We encountered a case of early onset PTLD after an unrelated HSCT following reduced-intensity conditioning with cyclophosphamide/fludarabine/thymoglobulin. A 24 year old patient with severe aplastic anemia developed multiple lymphadenopathies at day 95 after HSCT. The excisional biopsy revealed HL-like PTLD, which tested positive to immunohistochemical staining for the EBV. The Ann Arbor stage was IIA. Immunosuppressive agents were discontinued for 2 weeks in order to induce a graft-versus-lymphoma effect without a response. A total 4 cycles of chemotherapy with doxorubicin (adriamycin)/bleomycin/ vinblastine/dacarbazine (ABVD) and radiotherapy (total dosage 3,400 cGy) were then carried out. The response to salvage treatment was complete remission. The patient showed no evidence of the disease at the follow-up performed 32 months after HSCT.
References
1. Park JH, Park JS, Kim CK, et al. Two cases of posttransplantation lymphoproliferative disorders in recipients of liver tranaplantation. Korean J Med. 1998; 56:399–402.
2. Chang Sunhee. Hugh JH, Kim DJ, et al. Posttransplantation lymphoproliferative disorder-A report of 4 cases–. Korean J Pathol. 2002; 36:45–50.
3. Semakula B, Rittenbach JV, Wang J. Hodgkin lymphoma-like posttransplantation lymphoproliferative disorder. Arch Pathol Lab Med. 2006; 130:558–60.
4. Ho M, Miller G, Atchison RW, et al. Epstein-Barr virus infections and DNA hybridization studies in posttransplantation lymphoma and lymphoproliferative lesions: the role of primary infection. J Infect Dis. 1985; 152:876–86.

5. Ho M, Jaffe R, Miller G, et al. The frequency of Epstein-Barr virus infection and associated lymphoproliferative syndrome after transplantation and its manifestations in children. Transplantation. 1988; 45:719–27.

6. Harris NL, Swerdlow SH, Frizzera G, Knowles DM. Posttransplant lymphoproliferative disorders. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press. 2006. 264–269. World Health Organization Classification of Tumours.
7. Loren AW, Porter DL, Stadtmauer EA, Tsai DE. Posttransplant lymphoproliferative disorder: a review. Bone Marrow Transplant. 2003; 31:145–55.

8. Frizzera G, Hanto DW, Gajl-Peczalska KJ, et al. Polymorphic diffuse B-cell hyperplasias and lymphomas in renal transplant recipients. Cancer Res. 1981; 41:4262–79.
9. Ghobrial IM, Habermann TM, Macon WR, et al. Differences between early and late posttransplant lymphoproliferative disorders in solid organ transplant patients: are they two different diseases? Transplantation. 2005; 79:244–7.

10. Knowles DM, Cesarman E, Chadburn A, et al. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood. 1995; 85:552–65.

11. Randhawa PS, Magnone M, Jordan M, Shapiro R, Demetris AJ, Nalesnik M. Renal allograft involvement by Epstein-Barr virus associated post-transplant lymphoproliferative disease. Am J Surg Pathol. 1996; 20:563–71.

12. Choi SH, Hahn HJ, Kim YS, Park K. Four cases of posttransplant lymphoproliferative disease (PTLD) following renal transplantation. J Korean Soc Transplant. 2000; 14:101–8.
13. Chadburn A, Cesarman E, Knowles DM. Molecular pathology of posttransplantation lymphoproliferative disorders. Semin Diagn Pathol. 1997; 14:15–26.
Fig. 1.
Bone marrow finding at diagnosis of severe aplastic anemia (cellularity 10%, H&E stain, ×40).
Fig. 2.
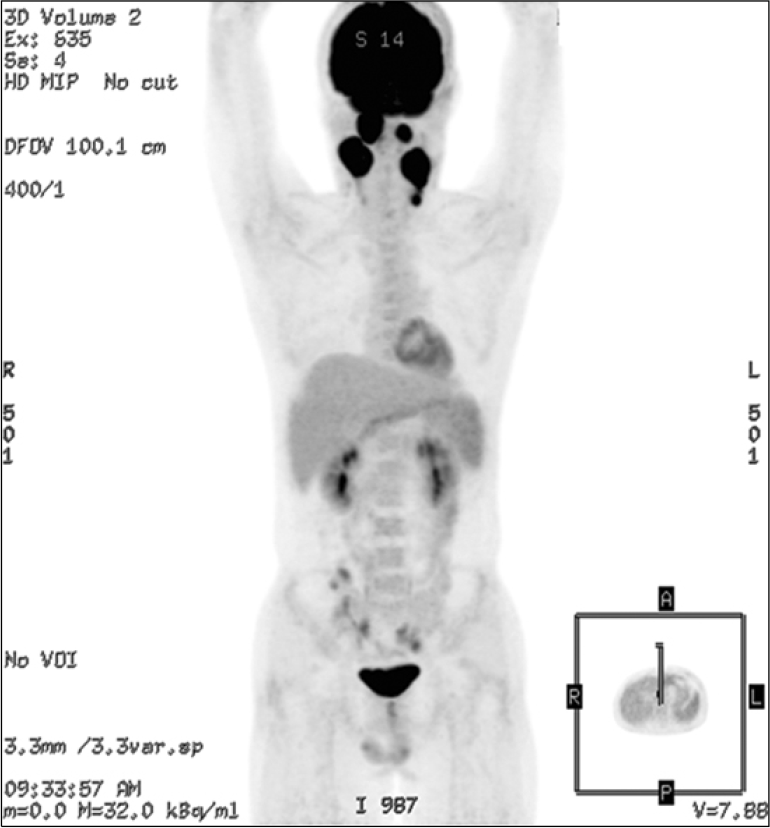
CT scan of neck at initial diagnosis of PTLD. Multiple enlarged cervical lymph nodes (arrows) with heterogeneous enhancement and relatively clear perinodal fat plane at both neck and lateral group of left retropharyngeal lymph node.
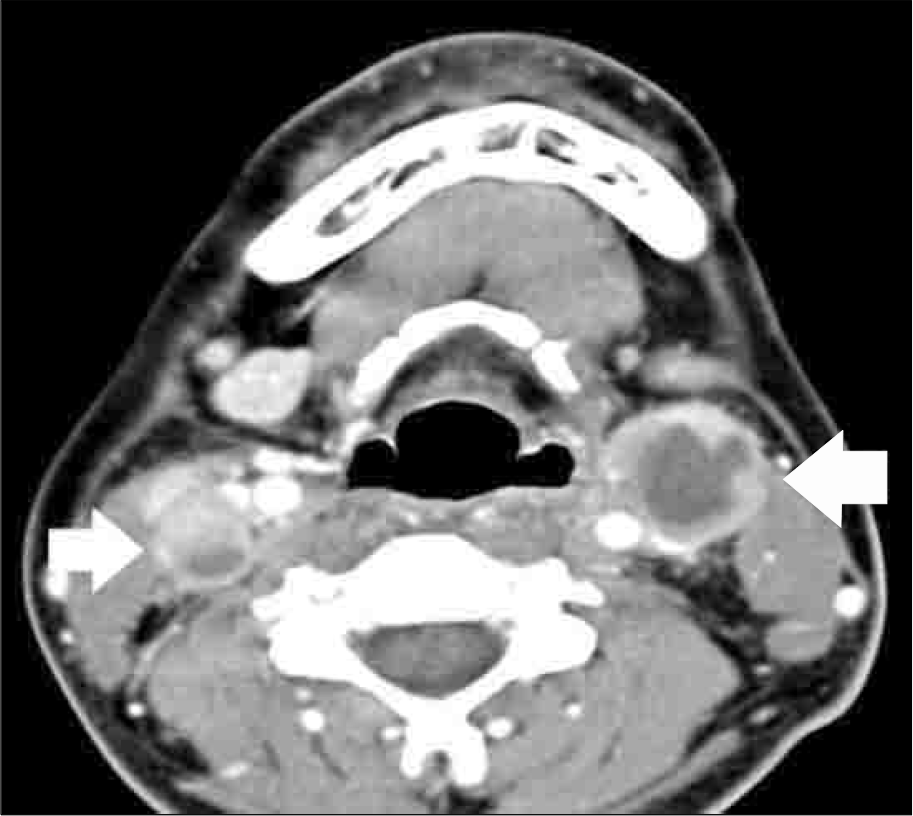
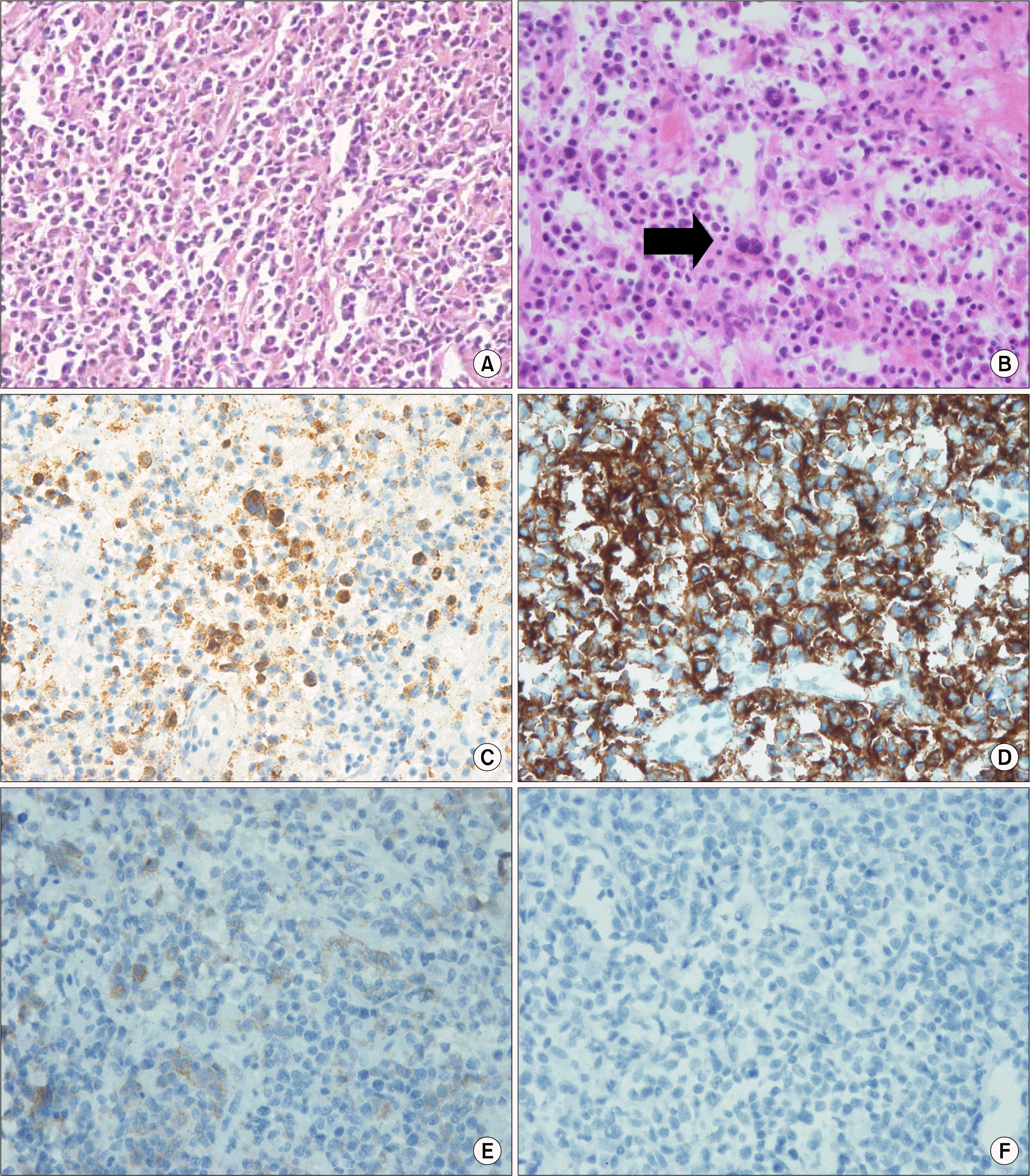
Fig. 3.
Pathologic findings of neck lymph node biopsy. (A) B lymphocyte maturation with small lymphocyte, medium size lymphoid cell, plasma cell, and immunoblast (H&E stain). (B) RS cell-like atypical immunoblast (arrow) and atypical lymphoid cells (H&E stain). (C) Positive for EBV in situ hybridization. (D) Positive for CD20 stain (B lymphocyte marker) within small lymphocyte, Reed Sternberg cell-like immunoblast and atypical lymphoid cells. (E) Positive for CD30 stain. (F) Negative for CD15 stain.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download