Abstract
Background
Iron overload, primarily related to RBC transfusions, is a relatively common complication in hematopoietic stem cell transplant (HSCT) recipients. There are emerging data from retrospective studies that iron overload can significantly increase the risk of nonrelapse mortality after allogeneic HSCT.
Methods
One hundred and five children who received allogeneic HSCT between Jan 2004 and Feb 2009 at Asan Medical Center were analyzed. For indirect estimation of body iron stores, we measured serum ferritin serially in HSCT recipients at pre-transplant, 3 months and 1 year post-transplant. We also analyzed prevalence of hyperferritinemia, correlation of iron overload and transplant-related outcomes and complications.
Results
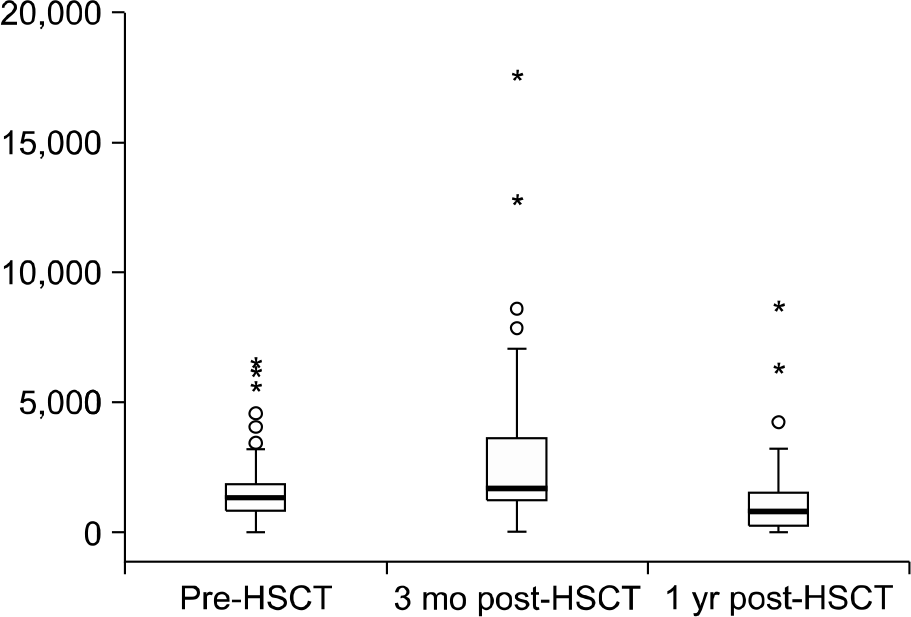
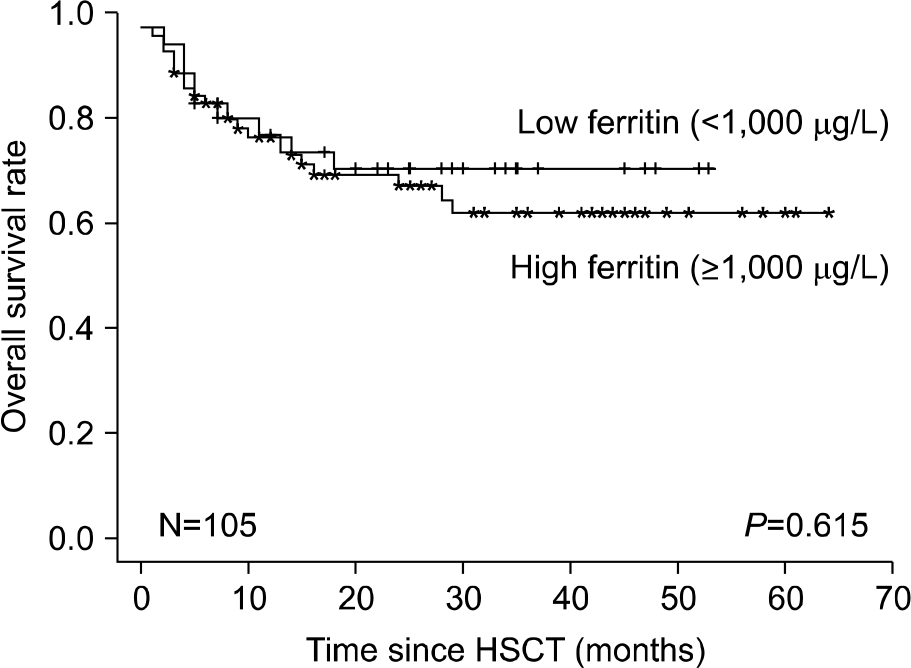
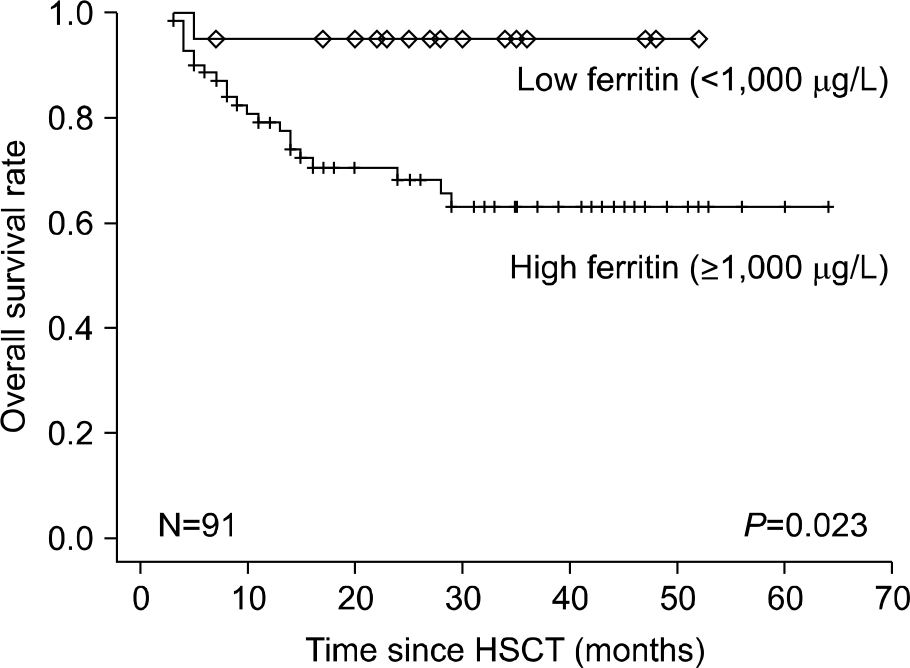
The prevalence of hyperferritinemia (≥1,000 μg/L) at pre-HSCT, 3 months and 1 year post-HSCT were 66.7% (70/105), 78% (71/91) and 40.9% (27/66), respectively. Children with hyper-ferritinemia (≥1,000 μg/L) at 3 months post-HSCT had worse 2-year OS (79% vs 95%; P=0.023) than those in the low ferritin group (<1,000 μg/L). Very high levels (VHL) of ferritin (≥3,000 μg/L) at 3 months post-HSCT were associated with increased incidence of treatment related mortality (23% vs 2%, P=0.001) and acute graft-versus-host disease (54% vs 26%, P=0.007) in univariate analysis. VHL of ferritin remained significant in multivariate analysis.
Conclusion
Hyperferritinemia at 3 months post-HSCT had adverse impact for transplantation outcome in patients undergoing allogeneic stem cell transplantation. These results suggest that the screening and adequate treatment of iron overload in HSCT recipients might be helpful to improve the HSCT outcomes.
References
1. Chotsampancharoen T, Gan K, Kasow KA, Barfield RC, Hale GA, Leung W. Iron overload in survivors of childhood leukemia after allogeneic hematopoietic stem cell transplantation. Pediatr Transplantation. 2009; 13:348–52.

2. de Witte T. The role of iron in patients after bone marrow transplantation. Blood Rev. 2008; 22(Suppl 2):22–8.

3. Kamble RT, Selby GB, Mims M, Kharfan-Dabaja MA, Ozer H, George JN. Iron overload manifesting as apparent exacerbation of hepatic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006; 12:506–10.

4. Lichtman SM, Attivissimo L, Goldman IS, Schuster MW, Buchbinder A. Secondary hemochromatosis as a longterm complication of the treatment of hematologic malignancies. Am J Hematol. 1999; 61:262–4.

5. Altès A, Remacha AF, Sureda A, et al. Iron overload might increase transplant-related mortality in haematopoietic stem cell transplantation. Bone Marrow Transplant. 2002; 29:987–9.

6. Majhail NS, DeFor T, Lazarus HM, Burns LJ. High prevalence of iron overload in adult allogeneic hematopoietic cell transplant survivors. Biol Blood Marrow Transplant. 2008; 14:790–4.

7. Kataoka K, Nannya Y, Hangaishi A, et al. Influence of pretransplantation serum ferritin on nonrelapse mortality after myeloablative and nonmyeloablative allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009; 15:195–204.
8. Pullarkat V, Blanchard S, Tegtmeier B, et al. Iron overload adversely affects outcome of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2008; 42:799–805.

9. Kim YR, Kim JS, Cheong JW, Song JW, Min YH. Transfusion-associated iron overload as an adverse risk factor for transplantation outcome in patients undergoing reduced-intensity stem cell transplantation for myeloid malignancies. Acta Haematol. 2008; 120:182–9.

10. Kamble R, Mims M. Iron-overload in longterm survivors of hematopoietic transplantation. Bone Marrow Transplant. 2006; 37:805–6.

11. Majhail NS, Lazarus HM, Burns LJ. Iron overload in hematopoietic cell transplantation. Bone Marrow Transplant. 2008; 41:997–1003.

12. Armand P, Kim HT, Cutler CS, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007; 109:4586–8.

13. Or R, Matzner Y, Konijn AM. Serum ferritin in patients undergoing bone marrow transplantation. Cancer. 1987; 60:1127–31.

14. Halonen P, Mattila J, Suominen P, Ruuska T, Salo MK, Mäkipernaa A. Iron overload in children who are treated for acute lymphoblastic leukemia estimated by liver siderosis and serum iron parameters. Pediatrics. 2003; 111:91–6.

15. Sucak GT, Yegin ZA, Ozkurt ZN, Aki SZ, Karakan T, Akyol G. The role of liver biopsy in the workup of liver dysfunction late after SCT: is the role of iron overload underestimated? Bone Marrow Transplant. 2008; 42:461–7.

16. Harrison P, Neilson JR, Marwah SS, Madden L, Bareford D, Milligan DW. Role of non-transferrin bound iron in iron overload and liver dysfunction in long term survivors of acute leukaemia and bone marrow transplantation. J Clin Pathol. 1996; 49:853–6.

17. Maradei SC, Maiolino A, de Azevedo AM, Colares M, Bouzas LF, Nucci M. Serum ferritin as risk factor for sinusoidal obstruction syndrome of the liver in patients undergoing hematopoietic stem cell transplantation. Blood. 2009; 114:1270–5.

18. Zhu KE, Hu JY, Zhang T, Chen J, Zhong J, Lu YH. Incidence, risks, and outcome of idiopathic pneumonia syndrome early after allogeneic hematopoietic stem cell transplantation. Eur J Haematol. 2008; 81:461–6.

19. Fukuda T, Hackman RC, Guthrie KA, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood. 2003; 102:2777–85.

20. Mahindra A, Bolwell B, Sobecks R, et al. Elevated pretransplant ferritin is associated with a lower incidence of chronic graft-versus-host disease and inferior survival after myeloablative allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2009; 146:310–6.

21. Malcovati L. Impact of transfusion dependency and secondary iron overload on the survival of patients with myelodysplastic syndromes. Leuk Res. 2007; 31(Suppl 3):2–6.

22. Rose C, Ernst O, Hecquet B, et al. Quantification by magnetic resonance imaging and liver consequences of post-transfusional iron overload alone in long term survivors after allogeneic hematopoietic stem cell transplantation (HSCT). Haematologica. 2007; 92:850–3.
23. Carreras E. Venoocclusive disease of the liver after hemopoietic cell transplantation. Eur J Haematol. 2000; 64:281–91.

24. Cesaro S, Pillon M, Talenti E, et al. A prospective survey on incidence, risk factors and therapy of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Haematologica. 2005; 90:1396–404.
Fig. 4.
Cumulative incidence of treatment related mortality (A) and acute graft-versus-host disease (B) according to serum ferritin level at 3 months post-HSCT.
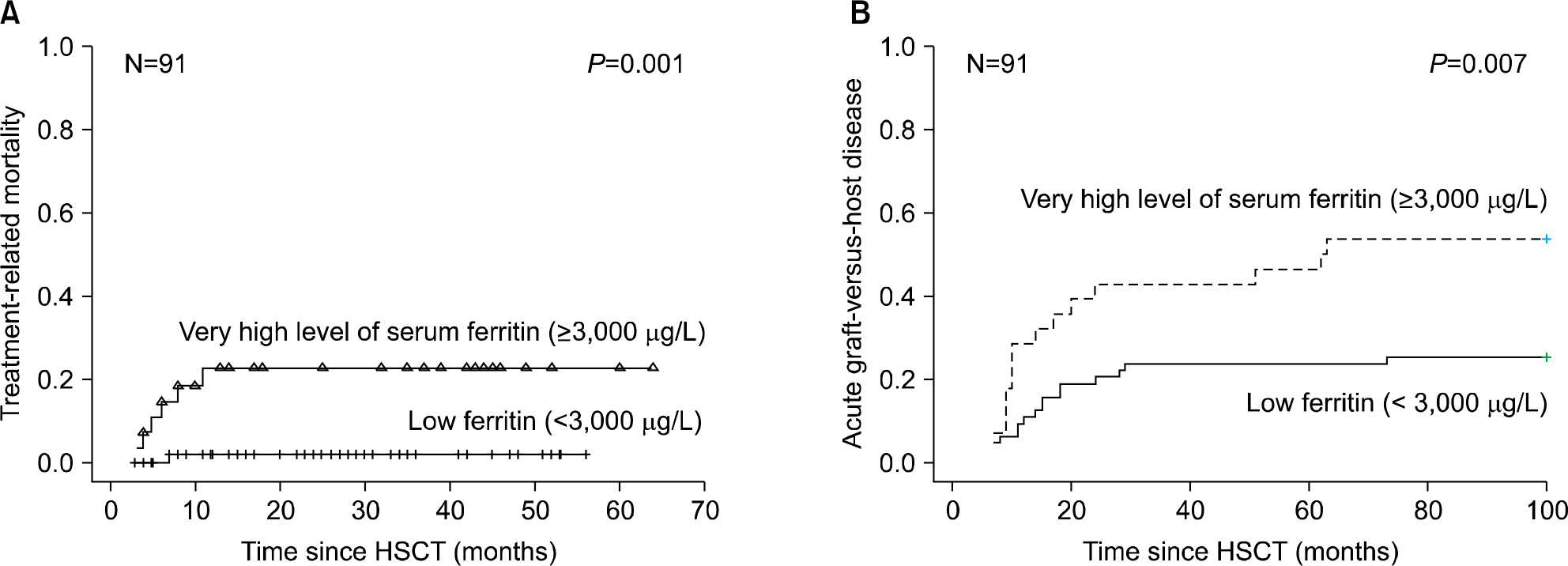
Table 1.
Clinical characteristics of 105 pediatric allogeneic HSCT recipients
Abbreviations: AML, indicates acute myeloblastic leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; ABL, acute biphenotypic leukemia; CML, chronic myeloblastic leukemia; HLH, hemophagoctic lymphohistiocytosis; VOD, venoocclusive disease; aGVHD, acute graft-versus-host-disease; cGVHD, chronic graft-versus-host disease.
Table 2.
Number of packed red cell transfusions by serum ferritin level
| Serum ferritin | |||
|---|---|---|---|
| Low (<1,000 μg/L) | High (≥1,000 μg/L | P-value | |
| Pre-HSCT | 6.7±4.3 | 16.8±13.3 | <0.005 |
| 3 months post-HSCT | 3.1±1.8 | 6.0±5.4 | 0.021 |
| 1 year post-HSCT | 3.9±3.1 | 9.9±9.0 | <0.005 |
Table 3.
Clinical characteristics of pediatric allogeneic HSCT recipients according to serum ferritin level at pre-HSCT and 3 months, 1 year post-HSCT
Table 4.
Complications after HSCT according to serum ferritin level




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download