Abstract
Background
Vascular endothelial growth factor (VEGF) plays an essential role in promoting angiogenesis during tumor development. In addition, VEGF can mediate the inflammatory response in tumors. VEGF increases the level of neutrophil migration by upregulating interleukin-8 (IL-8) in endothelial cells in vitro. However, it is unclear if VEGF can mediate IL-8 production in vivo.
Methods
To address this issue, this study examined the effect of VEGF on IL-8 production in vivo using an adenovirus transduction and mouse ear assay.
Results
Adenovirus-encoded VEGF (VEGF-Ad) increased the level of IL-8 production in endothelial cells in vitro compared to the control-adenovirus (CTL-Ad). The mouse ear assay showed that VEGF-Ad increased the level of IL-8 production in the endothelium. Immunohistochemistry showed that the IL-8 proteins were expressed in the vasculature within a human glioblastoma, which is known to strongly express VEGF.
Go to : 
References
1. Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2004; 23:1011–27.

2. Cai J, Ahmad S, Jiang WG, et al. Activation of vascular endothelial growth factor receptor-1 sustains angiogenesis and Bcl-2 expression via the phosphatidylinositol 3-kinase pathway in endothelial cells. Diabetes. 2003; 52:2959–68.

3. Senger DR, Perruzzi CA, Feder J, Dvorak HF. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986; 46:5629–32.
4. Berkman RA, Merrill MJ, Reinhold WC, et al. Expression of the vascular permeability factor/vascular endothelial growth factor gene in central nervous system neoplasms. J Clin Invest. 1993; 91:153–9.

5. Boocock CA, Charnock-Jones DS, Sharkey AM, et al. Expression of vascular endothelial growth factor and its receptors flt and KDR in ovarian carcinoma. J Natl Cancer Inst. 1995; 87:506–16.
6. Brown LF, Berse B, Jackman RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol. 1995; 26:86–91.

7. Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993; 362:841–4.

8. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997; 18:4–25.

9. Thomas AL, Morgan B, Drevs J, et al. Vascular endothelial growth factor receptor tyrosine kinase inhibitors: PTK787/ZK 222584. Semin Oncol. 2003; 3:32–8.

10. Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol. 2007; 2:251–75.

11. Falanga A, Panova-Noeva M, Russo L. Procoagulant mechanisms in tumour cells. Best Pract Res Clin Haematol. 2009; 22:49–60.

12. Nelson PJ, Krensky AM. Chemokines, chemokine receptors, and allograft rejection. Immunity. 2001; 14:377–86.

13. Marumo T, Schini-Kerth VB, Busse R. Vascular endothelial growth factor activates nuclear factor-kappaB and induces monocyte chemoattractant protein-1 in bovine retinal endothelial cells. Diabetes. 1999; 48:1131–7.

14. Lee TH, Avraham H, Lee SH, Avraham S. Vascular endothelial growth factor modulates neutrophil transendothelial migration via upregulation of interleukin-8 in human brain microvascular endothelial cells. J Biol Chem. 2002; 277:10445–51.

15. Lee TH, Seng S, Sekine M, et al. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through intenally expressed VEGFR1/FLT1. PLoS Med. 2007; 4:e186.
Go to : 
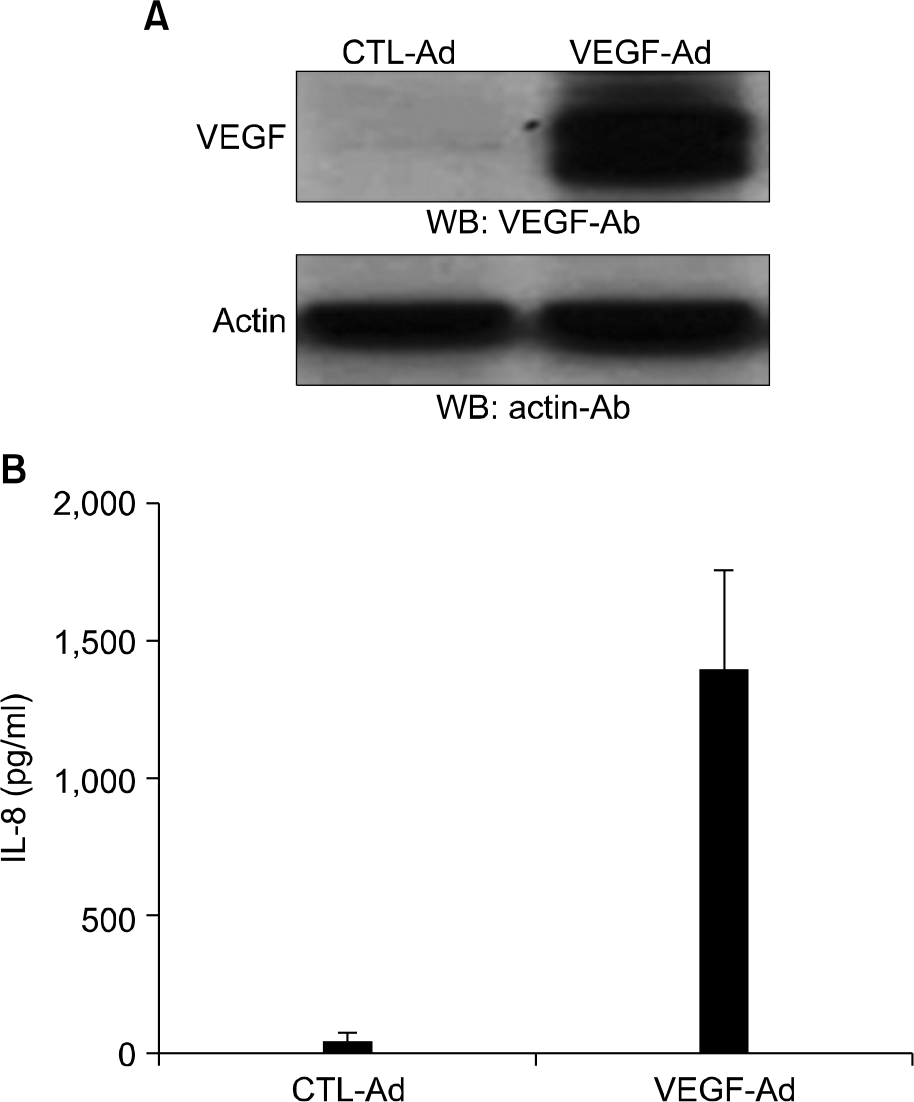 | Fig. 1.IL-8 production by VEGF-Ad infection in HUVECs. HUVECs were grown subconfluently in 6-well plates and in-fected with 10 MOI of VEGF-Ad or CTL-Ad. After incubation of 3 days, cells were lysed and VEGF expression was examined by western blot analysis (A). Alternatively, super-natants were harvested and IL-8 proteins were measured by using ELISA (B). |
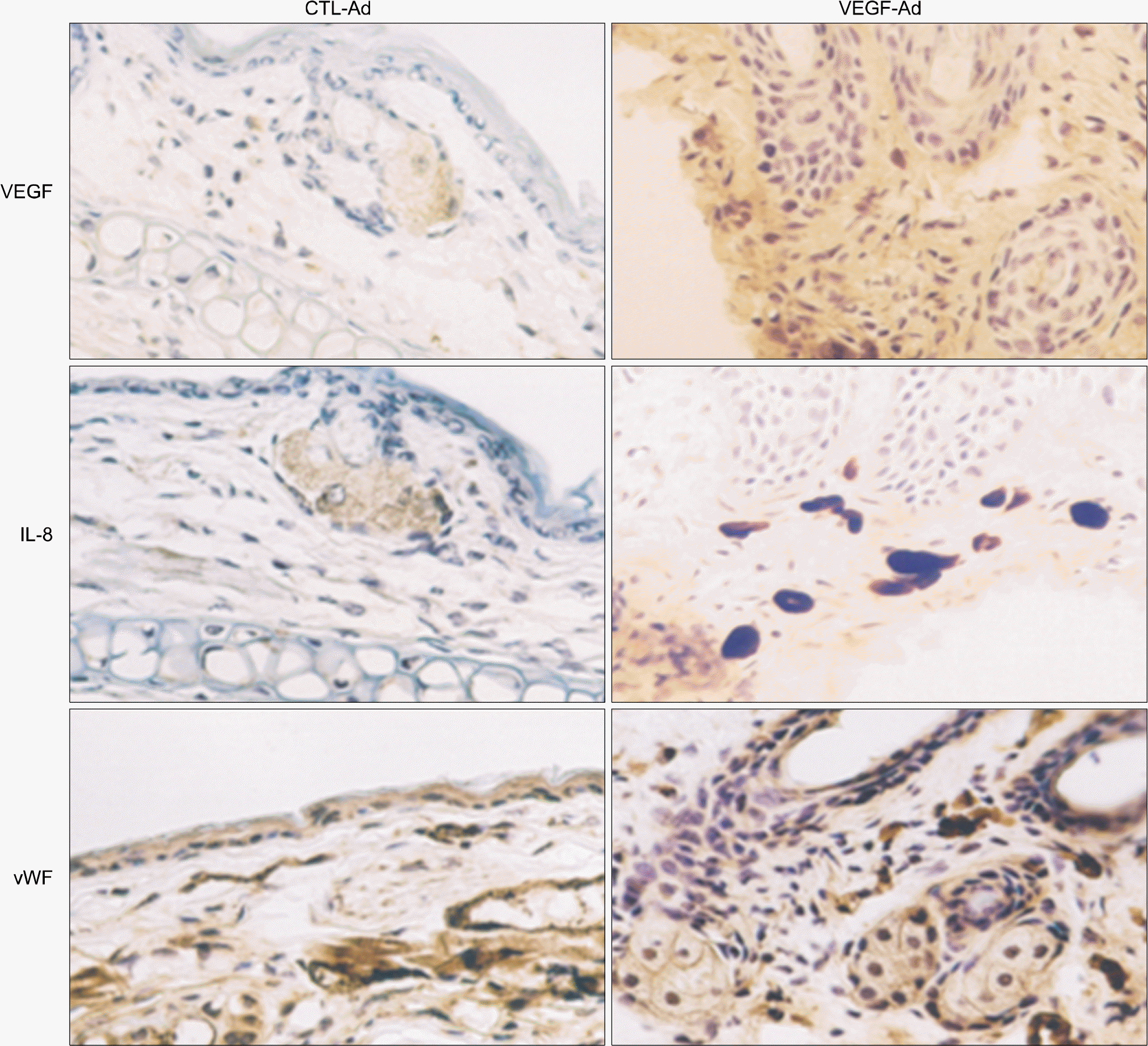 | Fig. 2.IL-8 production by VEGF-Ad infection in mouse ears. Mouse ears were infected with 100 MOI of VEGF-Ad or CTL-Ad. After 7 days, mice were sacrificed and ears were excised to be frozen. The ears were cut into 5 mm and fixed with cold methanol. Immunohistochemistry was performed as described under “methods”. vWF, von Willebrand factor. |
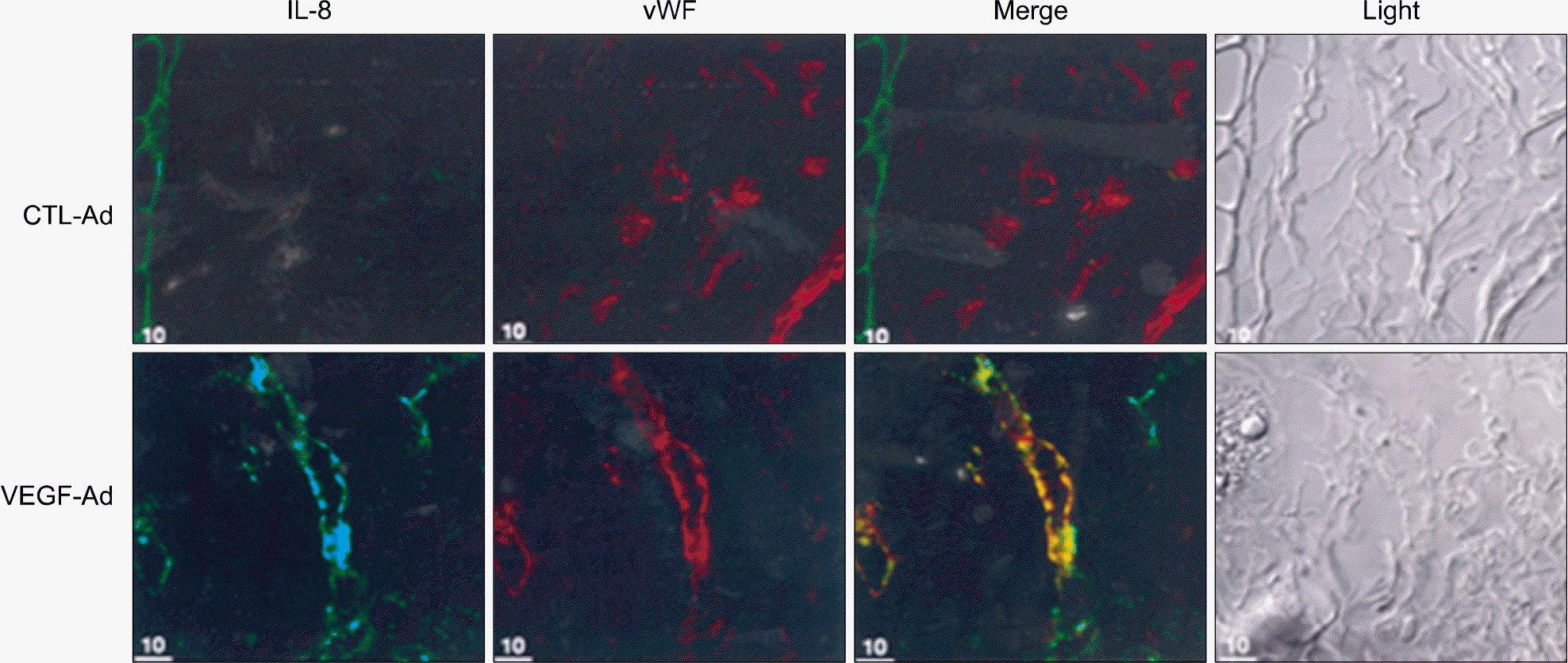 | Fig. 3.IL-8 and vWF colocalization in the VEGF-Ad infected mouse ears. Mouse ears were infected with 100 MOI of VEGF-Ad or CTL-Ad. After 7 days, mice were sacrificed and ears were excised to be frozen. The ears were cut into 5 μm and fixed with cold methanol. Colocalization of IL-8 and vWF was observed by using confocal microscopy. vWF, von Willebrand factor. |
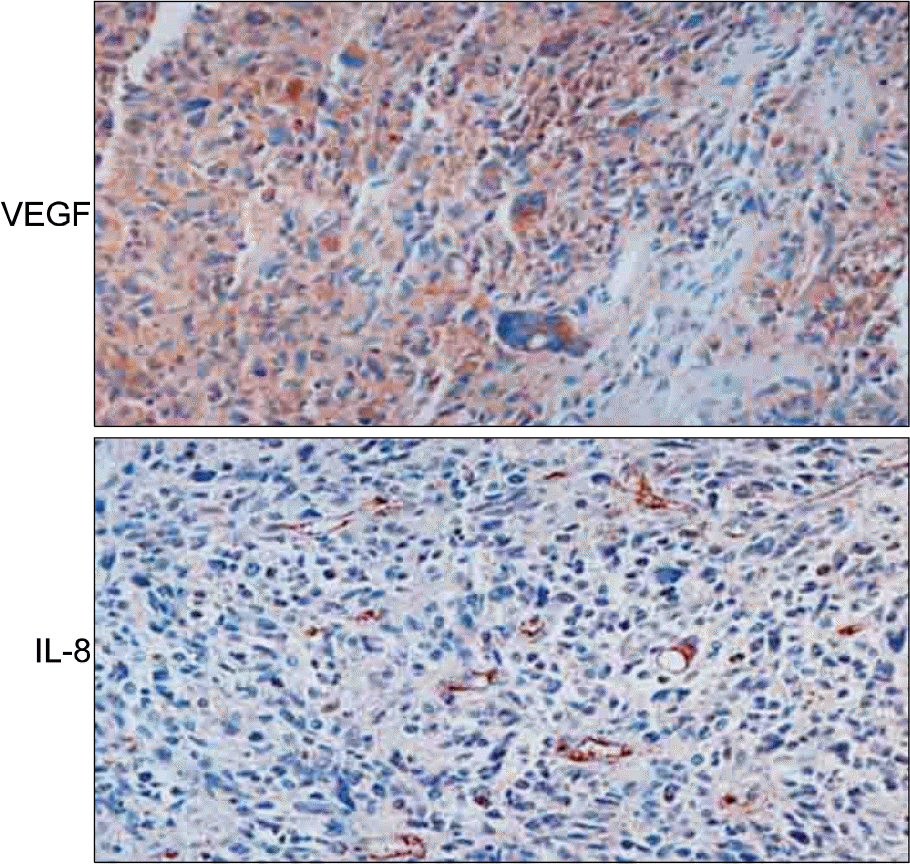 | Fig. 4.VEGF and IL-8 expression in human glioblastoma. The tissues were deparaffinized and fixed with 10% formaldehyde, then treated with 0.1% trypsin for 30 min to improve antigen accessibility. After blocking nonspecific binding to secondary antibodies by treatment with blocking solution (Dako Inc.) for 1 h, the tissues were incubated overnight at 4oC with primary antibodies against VEGF or IL-8 polyclonal antibodies. The tissues were washed and incubated with horseradish peroxidase-conjugated secondary antibodies (Vector Laboratories) for 1 h. After washing, tissues were stained using the Vectastain ABC kit. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download