Abstract
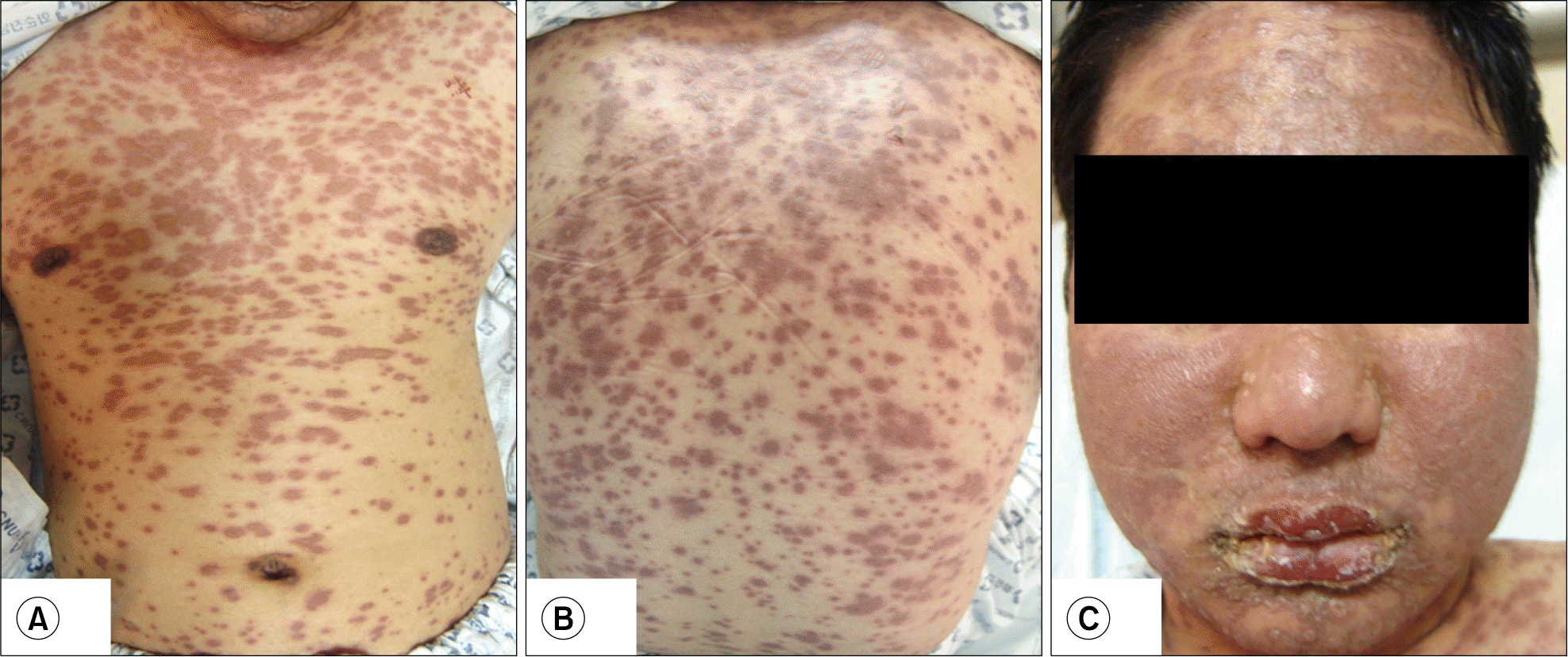
Rituximab is a chimeric monoclonal antibody that specifically targets the CD20 molecule on the B cell surface. Although rituximab was originally introduced for the treatment of lymphoid neoplasms such as non-Hodgkin's lymphoma (NHL) and chronic lymphocytic leukemia (CLL), it is now emerging as an effective and relatively safe therapeutic option for the patients with refractory immune thrombocytopenic purpura (ITP). We report here on a case of life-threatening toxic epidermal necrolysis (TEN) that was related with the use of rituximab in a patient with refractory ITP. The patient developed extensive erythematous papules and bullous lesions on his whole body associated with fever and visual disturbance during the second cycle of rituximab. The rituximab was discontinued and high dose intravenous immunoglobu-line and steroid were administrated. Four weeks later, he fully recovered without any sequelae. A review of the literature reveals this to be the first reported case of TEN associated with rituximab injection in Korea.
References
1. Maloney DG, Smith B, Rose A. Rituximab: mechanism of action and resistance. Semin Oncol. 2002; 29:2–9.

2. Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large B-cell lymphoma. N Engl J Med. 2002; 346:235–42.
3. Pasa S, Altintas A, Cil T, Danis R, Ayyildiz O. The efficacy of rituximab in patients with splenectomized refractory chronic idiopathic thrombocythopenic purpura. J Thromb Thrombolysis. 2009; 27:329–33.

4. Allen KP, Funk AJ, Mandrell TD. Toxic epidermal necrolysis in two rhesus macaques (Macaca mulatta) after administration of rituximab. Comp Med. 2005; 55:377–81.
5. Schütt P, Passon J, Ebeling P, et al. Ifosfamide, etoposide, cytarabine, and dexamethasone as salvage treatment followed by high-dose cyclophosphamide, melphalan, and etoposide with autologous peripheral blood stem cell transplantation for relapsed or refractory lymphomas. Eur J Haematol. 2007; 78:93–101.

6. Roujeau JC. The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: a clinical classification. J Invest Dermatol. 1994; 102:28S–30S.

7. Revuz JE, Roujeau JC. Advances in toxic epidermal necrolysis. Semin Cutan Med Surg. 1996; 15:258–66.

8. Prins C, Kerdel FA, Padilla RS, et al. Treatment of toxic epidermal necrolysis with high-dose intravenous immunoglobulins: multicenter retrospective analysis of 48 consecutive cases. Arch Dermatol. 2003; 139:26–32.
10. Maeng CH, Chin SO, Yang BH, et al. A case of organizing pneumonia associated with rituximab. Cancer Res Treat. 2007; 39:88–91.

11. Lee Y, Kyung SY, Choi SJ, et al. Two cases of interstitial pneumonitis caused by rituximab therapy. Korean J Intern Med. 2006; 21:183–6.

12. Yi JH, Kim SJ, Ahn HK, Lee SJ, Chang MH, Kim WS. Rituximab-induced acute thrombocytopenia: a case report and review of the literature. Med Oncol. 2009; 26:45–8.

13. Grillo-López AJ, Hedrick E, Rashford M, Benyunes M. Rituximab: ongoing and future clinical development. Semin Oncol. 2002; 29:105–12.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download