Abstract
Background:
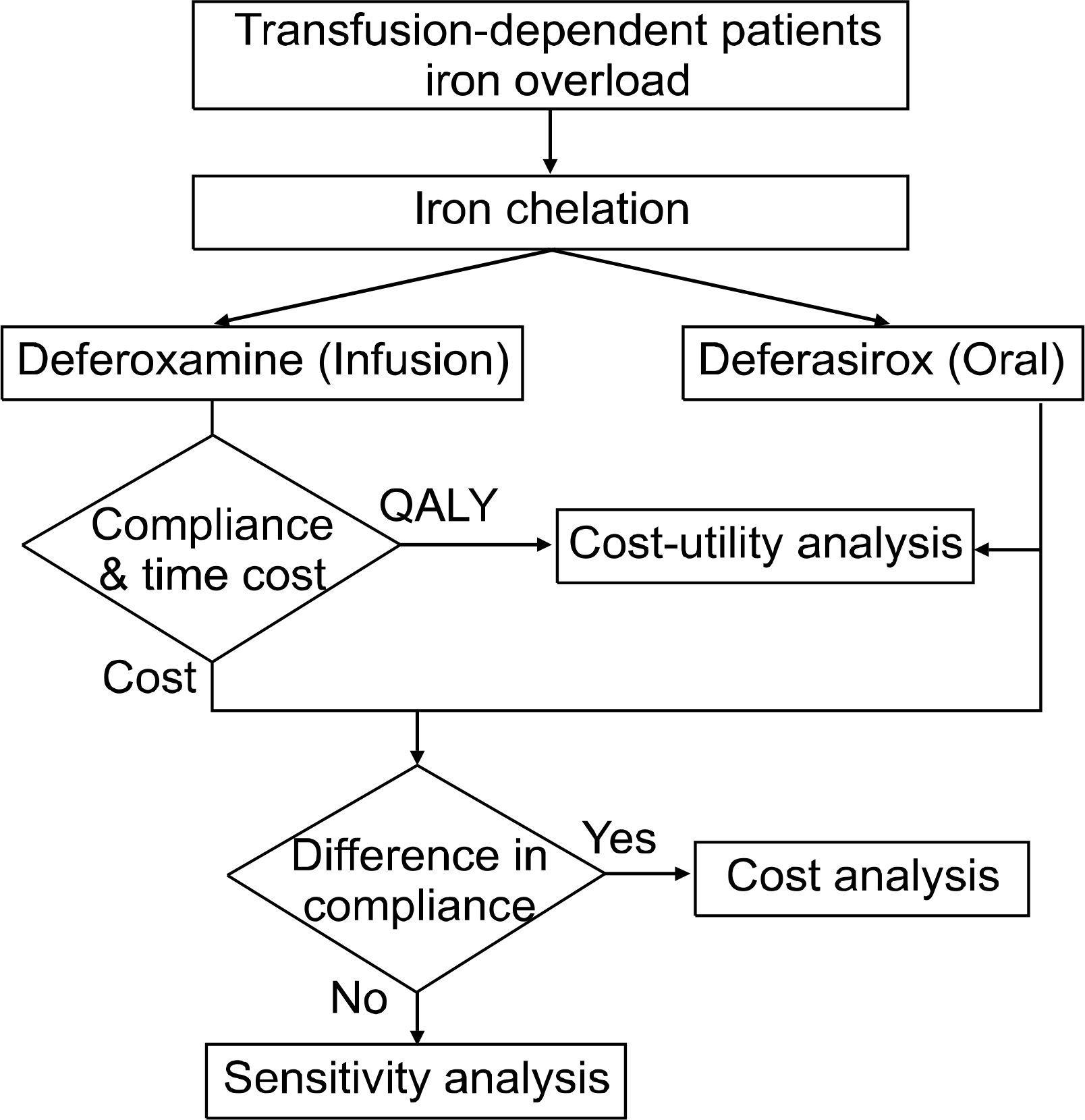
Patients with transfusional iron overload have relied on treatment with deferoxamine, a standard chelating agent. Deferoxamine is administered by intravenous or subcutaneous infusion over an 8∼12 hour period 5∼7 times per week; however, administration of deferoxamine may lead to poor compliance and reduced quality of life in patients. The use of deferasirox, a once daily oral chelation agent, was recently approved. We conducted an economic evaluation of these two iron-chelating medications in transfusion-dependent patients.
Methods:
The efficacy of oral deferasirox and infusion deferoxamine was assumed equal based on clinical trials of non-inferiority with the administration of 20mg/kg/day deferasirox versus 40mg/kg/day deferoxamine. Depending on the methods utilized for measuring administration time, travel time and convenience between the use of infusion and oral therapy, either cost analysis or cost-utility analysis was undertaken, respectively. Cost analysis included determination of direct medical costs (drug costs and administration costs), non-medical costs (travel costs), and indirect costs (productivity loss associated medical utilization). For cost utility analysis, the cost per QALYs (quality-adjusted life years) was calculated based on costs subtracting indirect costs (productivity loss) and gains of QALYs between the two agents.
Go to : 
REFERENCES
1). Cappellini MD., Cohen A., Piga A, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006. 107:3455–62.

3). Piga A., Galanello R., Forni GL, et al. Randomized phase II trial of deferasirox (Exjade, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overload. Haematologica. 2006. 91:873–80.
4). Neufeld EJ. Oral chelators deferasirox and deferiprone for transfusional iron overload in thalassemia major: new data, new questions. Blood. 2006. 107:3436–41.

5). Cappellini MD., Bejaoui M., Agaoglu L, et al. Prospective evaluation of patient-reported outcomes during treatment with deferasirox or deferoxamine for iron overload in patients with beta-thalassemia. Clin Ther. 2007. 29:909–17.
6). Vichinsky E., Pakbaz Z., Onyekwere O, et al. Patient-reported outcomes of deferasirox (Exjade? ICL670) versus deferoxamine in sickle cell disease patients with transfusional hemosiderosis. Substudy of a randomized open-label phase II trial. Acta Haematol. 2008. 119:133–41.
7). Osborne RH., De Abreu Lourenco R., Dalton A, et al. Quality of life relatedto oral versus subcutaneous iron chelation: a time trade-off study. Value in Health. 2007. 10:451–6.
8). Fischer R., Longo F., Nielsen P., Engelhardt R., Hider RC., Piga A. Monitoring long-term efficacy of iron chelation therapy by deferiprone and desferriox-amine in patients with beta-thalassaemia major: application of SQUID biomagnetic liver susceptometry. Br J Haematol. 2003. 121:938–48.
9). Delea TE., Sofrygin O., Thomas SK., Baladi JF., Phatak PD., Coates TD. Cost effectiveness of once-daily oralchelation therapy with deferasirox versus infusional deferoxamine in transfusion-dependent thalassaemia patients: US healthcare system perspective. Pharmacoeconomics. 2007. 25:329–42.
10). Canatan D., Temimhan N., Dincer N., Ozsancak A., Oğuz N., Temimhan M. Continuous desferoxamine infusion by an infusor in thalassaemia major. Acta Paediatr. 1999. 88:550–2.

11). Chan GC., Ng DM., Fong DY., Ha SY., Lau YL. Comparison of subcutaneous infusion needles for transfusion-dependent thalassemia patients by the intrapersonal cross-over assessment model. Am J Hematol. 2004. 76:398–404.

12). Sharma JB., Jain S., Mallika V, et al. A prospective, partially randomized study of pregnancy outcomes and hematologic responses to oral and intramuscular iron treatment in moderately anemic pregnant women. Am J Clin Nutr. 2004. 79:116–22.

13). Rossini M., Braga V., Gatti D., Gerardi D., Zamberlan N., Adami S. Intramuscular clodronate therapy in postmenopausal osteoporosis. Bone. 1999. 24:125–9.

14). Varsano I., Volovitz B., Horev Z, et al. Intramuscular ceftriaxone compared with oral amoxicillin-clavulanate for treatment of acute otitis media in children. Eur J Pediatr. 1997. 156:858.

15). JundtJW. Browne BA., Fiocco GP., Steele AD., Mock D. A comparison of low dose methotrexate bioavail-ability: oral solution, oral tablet, subcutaneous and intramuscular dosing. J Rheumatol. 1993. 20:1845–9.
Go to : 
Table 1.
Comparison of available iron chelators
| Parameter | DFO | DSX |
|---|---|---|
| Dosages (based on 50kg) | 2,000mg | 1,000mg |
| Utility value QALYs | 0.61 28.85 | 0.85 40.21 |
| Costs | 10,959 Won/500mg | 27,462 Won/500mg |
Table 2.
Costs of DFO and DSX (Unit: Won)
Table 3.
Results of cost analysis and cost-utility analysis
Table 4.
LYG according to the compliance with DFO and DSX
Table 5.
Sensitivity analysis for compliance with DFO and DSX: ICER (Unit: Won/LYG)
| Compliance with DSX | ||
|---|---|---|
| 74% (271 days) (LYG: 47.3) | 84% (308 days) (LYG: 57) | |
| Compliance with DFO 64% (166 infusions) (LYG: 28.4) | −683,917 | −378,676 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download