Abstract
Background:
Although the platelet count may not always correlate with the risk of thrombosis, there is evidence that a strict control of the platelet count decreases the incidence of thrombotic and hemorrhagic complications. However, it is difficult to select an appropriate platelet-lowering agent. This retrospective study was performed to assess the efficacy and adverse effect of the use of hydroxyurea and anagrelide for patients with essential thrombocythemia.
Methods:
Sixty patients with essential thrombocythemia received either hydroxyurea (n=30) or anagrelide (n=30). Early responses and adverse effects of hydroxyurea and anagrelide in the patients were retrospectively analyzed.
Results:
Treatment with anagrelide or hydroxyurea resulted in a rapid decrease of the platelet count within two weeks. The response rates after treatment with hydroxyurea and anagrelide were 83% and 77%, respectively. As compared with patients treated with hydroxyurea, patients treated with anagrelide presented with adverse effects such as headache palpitation was also frequently noticed (P=0.001). However, serious hemorrhage (n=2) and transformation to leukemia (n=1) occurred in patients treated with hydroxyurea.
Go to : 
REFERENCES
1). McCune JS., Liles D., Lindley C. Precipitous fall in platelet count with anagrelide: case report and critique of dosing recommendations. Pharmacotherapy. 1997. 17:822–6.
2). Silverstein MN., Tefferi A. Treatment of essential thrombocythemia with anagrelide. Semin Hematol. 1999. 36(1 Suppl 2):23–5.
3). Cortelazzo S., Viero P., Finazzi G., D'Emilio A., Rodeghiero F., Barbui T. Incidence and risk factors for thrombotic complications in a historical cohort of 100 patients with essential thrombocythemia. J Clin Oncol. 1990. 8:556–62.

4). Lahuerta-Palacios JJ., Bornstein R., Fernández-Debora FJ, et al. Controlled and uncontrolled thrombocytosis. Its clinical role in essential thrombocythemia. Cancer. 1998. 61:1207–12.
5). Millard FE., Hunter CS., Anderson M, et al. Clinical manifestations of essential thrombocythemia in young adults. Am J Hematol. 1990. 33:27–31.

6). Cortelazzo S., Finazzi G., Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythaemia and high risk of thrombosis. N Engl J Med. 1995. 332:1132–6.
7). Barbui T., Finazzi G., Dupuy E., Kiladjian JJ., Brière J. Treatment strategies in essential thrombocythaemia. A critical appraisal of various experiences in different centres. Leuk Lymphoma. 1996. 22(Suppl 1):149–60.
8). Tefferi A., Fonseca R., Pereira DL., Hoagland HC. A long-term retrospective study of young women with essential thrombocythemia. Mayo Clin Proc. 2001. 76:22–8.

9). Lengfelder E., Hochhaus A., Kronawitter U, et al. Should a platelet limit of 600∗109/L be used as a diagnostic criterion in essential thrombocythaemia? An analysis of the natural course including early stages. Br J Haematol. 1998. 100:15–23.
10). Sterkens Y., Preudhomme C., Laï JL, et al. Acute myeloid leukemia and myelodysplastic syndromes following essential thrombocythaemia treated with hydroxyurea: high proportion of cases with 17p depletion. Blood. 1998. 91:616–22.
11). Finazzi G., Ruggeri M., Rodeghiero F., Barbui T. Second malignancies in patients with essential throm-bocythaemia treated with busulphan and hydroxyurea: long-term follow-up of a randomized clinical trial. Br J Haematol. 2000. 110:577–83.

12). Mazur EM., Rosmarin AG., Sohl PA., Newton JL., Narendran A. Analysis of the mechanism of anagrelide-induced thrombocytopenia in humans. Blood. 1992. 79:1931–7.

13). Solberg LA Jr., Tefferi A., Oles KJ, et al. The effects of anagrelide on human megakaryocytopoiesis. Br J Haematol. 1997. 99:174–80.
14). Tang SS., Frojmovic MM. Inhibition of platelet function by antithrombotic agents which selectively inhibit low-cyclic 3',5'-adenosine monophosphate phosphodiesterase. J Lab Clin Med. 1980. 95:241–57.
15). Kim JY., Kim YJ., Chung BH, et al. Therapeutic efficacy of anagrelide for thrombocytosis. Korean J Hematol. 2003. 38:164–8.
16). Kim JY., Lee KS. Treatment of children with anagrelide for essential thrombocythemia. Korean J Pediatr Hematol-Oncol. 2004. 11:39–44.
17). Murphy S., Peterson P., Iland H., Laszlo J. Experience of the Polycythemia Vera Study Group with essential thrombocythemia: a final report on diagnostic criteria, survival, and leukemic transition by treatment. Semin Hematol. 1997. 34:29–39.
18). Anagrelide Study Group. Anagrelide, a therapy for thrombocythemic states: experience in 577 patients. Anagrelide Study Group. Am J Med. 1992. 92:69–76.
19). Bellucci S., Legrand C., Boval B., Drouet L., Caen J. Studies of platelet volume, chemistry and function in patients with essential thrombocythaemia treated with anagrelide. Br J Haematol. 1999. 104:886–92.

20). Cacciola RR., Francesco ED., Giustolisi R., Cacciola E. Effects of anagrelide on platelet factor 4 and vascular endothelial growth factor levels in patients with essential thrombocythemia. Br J Haematol. 2004. 126:885–6.

21). Bouchard BA., Tracy PB. Platelets, leukocytes, and coagulation. Curr Opin Hematol. 2001. 8:263–9.

22). Falanga A., Marchetti M., Evangelista V, et al. Polymorphonuclear leukocyte activation and hemostasis in patients with essential thrombocythemia and polycythemia vera. Blood. 2000. 96:4261–6.

23). Brun M., Bourdoulous S., Couraud PO., Elion J., Krishnamoorthy R., Lapoumeroulie C. Hydroxyurea downregulates endothelin-1 gene expression and upregulates ICAM-1 gene expression in cultured human endothelial cells. Pharmacogenomics J. 2003. 3:215–26.

Go to : 
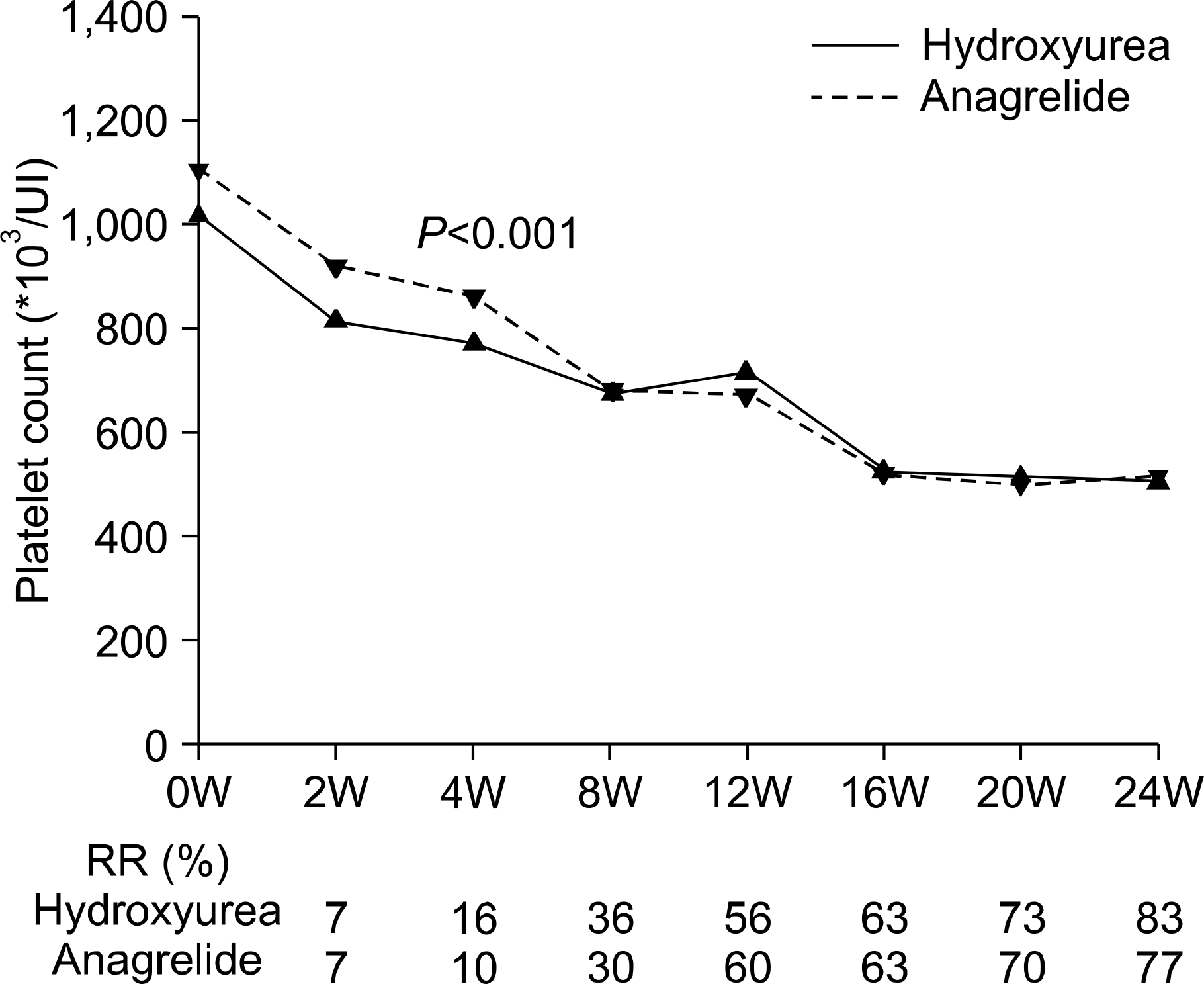 | Fig. 1Median of platelet count, according to weeks after treatment. Platelet counts were significantly different between two groups at two and four weeks. RR, response rate; W, week. |
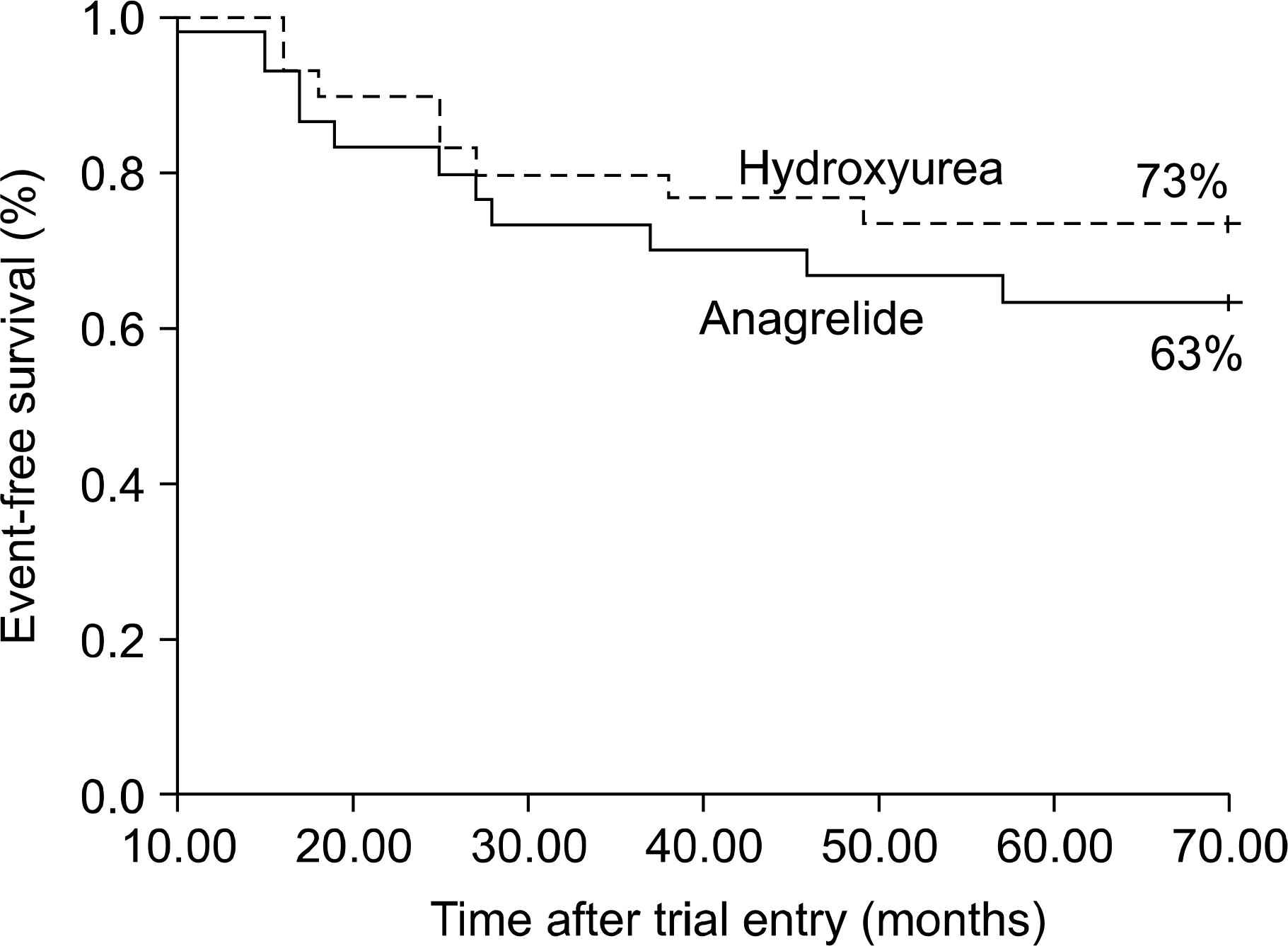 | Fig. 2Kaplan-Meier estimates of the event of thrombotic, hemorrhagic event and leukemic transformation. |
Table 1.
Baseline characteristics of patients
Table 2.
Treatment withdrawal and adverse events
Table 3.
Vascular and other event




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download