Abstract
Methods:
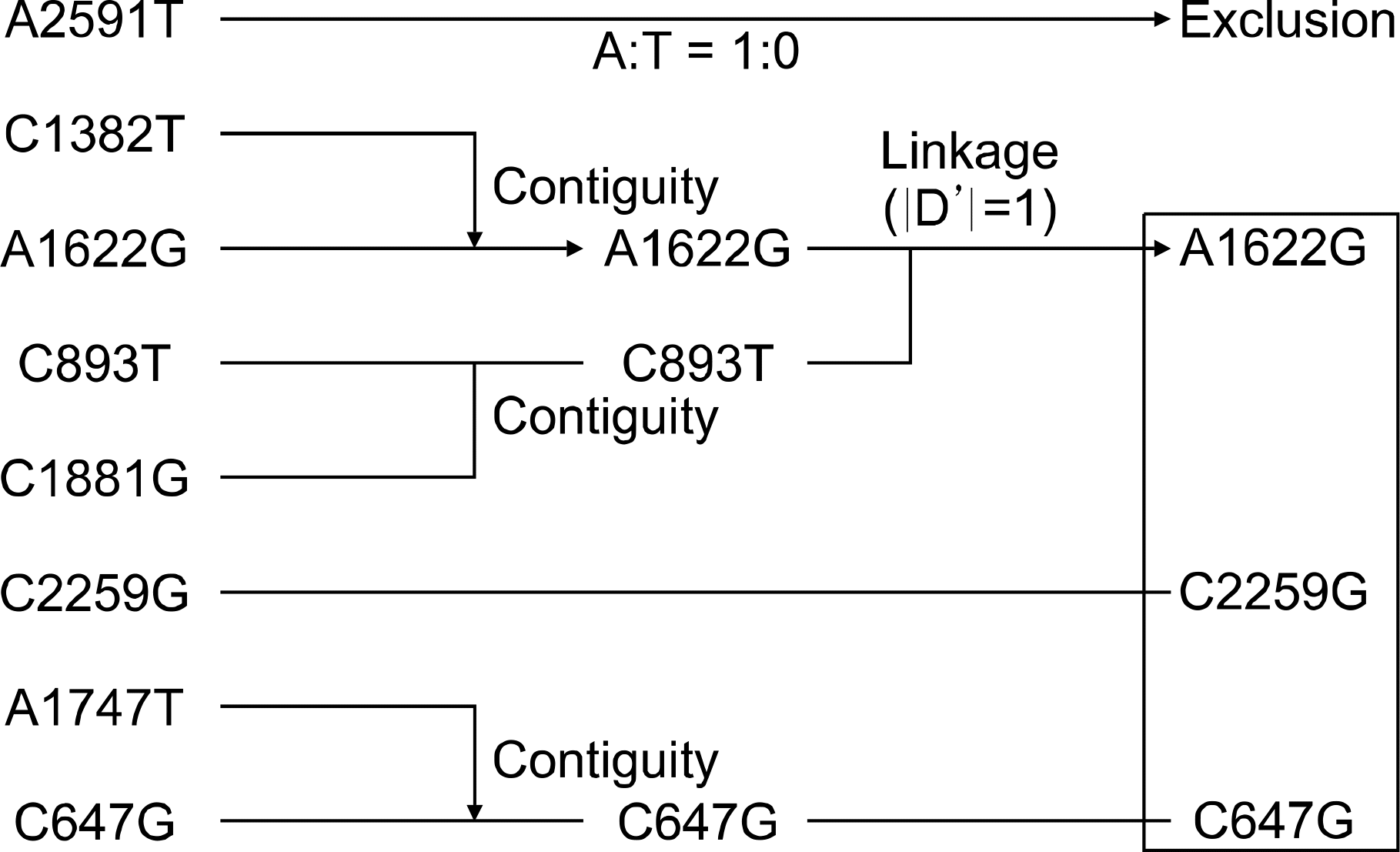
We tested eight sites of P2Y1 and studied the possible link between the presence of P2Y1 polymorphisms and the risk of is chemic vascular disease in a case-control study. The polymorphisms A1622G, C647G and C2259G were selected according to linkage disequilibrium. We evaluated 275 patients with is chemic cerebrovascular disease and 275 control subjects. We also evaluated 171 patients with acute myocardial infarction (AMI), 166 patients with unstable angina (UA), 173 patients with stable angina (SA) and 188 control subjects.
Results:
For the cerebrovascular disease patients, A1622G AA, AG [odds ratio (OR), 1.170; 95% confidence interval (CI), 0.784 to 1.748] and GG (OR, 1.031; 95% CI, 0.554 to 1.918) did not show any difference between the case and control subjects. C647G CC, CG (OR, 0.995; 95% CI, 0.639 to 1.550) and GG (OR, 1.012; 95% CI, 0.450 to 2.277) did not show any difference between the case and control subjects. C2259G CC, CG (OR, 0.619; 95% CI, 0.354 to 1.082) and GG did not show any difference between the case and control subjects. For coronary artery disease patients, C2259G GG, CG (for AMI patients OR, 0.880, 95% CI, 0.384 to 2.016; for UA patients, OR, 0.885, 95% CI, 0.410 to 1.911; for SA patients, OR, 1.156, 95% CI, 0.534 to 2.501) and CC did not show any difference between AMI, UA and SA patients and each control subject. C647G GG, CG (for AMI patients OR, 1.351, 95% CI, 0.731 to 2.497; for UA patients OR, 1.292, 95% CI, 0.723 to 2.309; for SA patients OR, 0.977, 95% CI, 0.530 to 1.803) and CC (for AMI patients OR, 0.355, 95% CI, 0.093 to 1.358; for UA patients OR, 0.645, 95% CI, 0.205 to 2.028; for SA patients OR, 0.385, 95% CI, 0.113 to 1.311) did not show any difference between AMI, UA and SA patients and each control subject. A1622G AA, AG (for AMI patients OR, 1.416, 95% CI, 0.786 to 2.549; for UA patients OR, 1.079, 95% CI, 0.611 to 1.904; for SA patients OR, 0.958, 95% CI, 0.529 to 1.732) and GG (for AMI patients OR, 0.525, 95% CI, 0.195 to 1.411; for UA patients OR, 0.568, 95% CI, 0.231 to 1.401; for SA patients OR, 0.441, 95% CI, 0.169 to 1.154) did not show any difference between AMI, UA and, SA patients and the control subjects.
Go to : 
REFERENCES
1). Rauch U., Osende JI., Fuster V., Badimon JJ., Fayad Z., Chesebro JH. Thrombus formation on atherosclerotic plaques: pathogenesis and clinical consequences. Ann Intern Med. 2001. 134:224–38.

2). Hetherington SL., Singh RK., Lodwick D., Thompson JR., Goodall AH., Samani NJ. Dimorphism in the P2Y1 ADP receptor gene is associated with increased platelet activation response to ADP. Arterioscler Thromb Vasc Biol. 2005. 25:252–7.

3). Yamada S., Akita H., Kanazawa K, et al. T102C polymorphism of the serotonin (5-HT) 2A receptor gene in patients with nonfatal acute myocardial infarction. Atherosclerosis. 2000. 150:143–8.

4). Kim GY. Genetic polymorphism of a platelet glycoprotein IIIa as an inherited risk factor for coronary artery disease in Koreans. Korean Circ J. 1997. 27:1082–6.
5). Park S., Park HY., Park C, et al. Association of the gene polymorphisms of platelet glycoprotein Ia and IIb/IIIa with myocardial infarction and extent of coronary artery disease in the Korean population. Yonsei Med J. 2004. 45:428–34.

6). Pyo MK., Yun-Choi HS., Hong YJ. Apparent heterogeneous responsiveness of human platelet rich plasma to catecholamines. Platelets. 2003. 14:171–8.

7). Kambayashi J., Shinoki N., Nakamura T, et al. Prevalence of impaired responsiveness to epinephrine in platelets among Japanese. Thromb Res. 1996. 81:85–90.

8). Yun-Choi HS., Park KM., Pyo MK. Epinephrine induced platelet aggregation in rat platelet-rich plasma. Thromb Res. 2000. 100:511–8.

9). Kim PJ., Chang K., Koh YS, et al. Functional polymorphism in the promoter region of matrix metal-loproteinase-9 is strongly associated with acute myocardial infarction. Korean Circ J. 2005. 35:192–6.

10). Jung CH., Rhee EJ., Kim SY, et al. Association between two SNPs (+45T>G and +276G>T) of the adiponectin gene and coronary artery diseases. Korean J Med. 2006. 70:393–401.
11). Kim JM., Lee YY., Koo YK, et al. Association between platelet polymorphism and cardiovascular diseases in the Korean population. In: 15th Congress of the Korean Society on Thrombosis and Hemostasis;2005. 12.02.: The Korean Journal of Thrombosis and Hemostasis; 2005;12: 113.
12). Di Castelnuovo A., de Gaetano G., Donati MB., Iacoviello L. Platelet glycoprotein receptor IIIa polymorphism PLA1/PLA2 and coronary risk: a meta-analysis. Thromb Haemost. 2001. 85:626–33.
13). Santoso S., Kunicki TJ., Kroll H., Haberbosch W., Gardemann A. Association of the platelet glycoprotein Ia C807T gene polymorphism with nonfatal myocardial infarction in younger patients. Blood. 1999. 93:2449–53.

14). Gonzalez-Conejero R., Lozano ML., Rivera J, et al. Polymorphisms of platelet membrane glycoprotein Ib alpha associated with arterial thrombotic disease. Blood. 1998. 92:2771–6.
15). Durante-Mangoni E., Davies GJ., Ahmed N., Ruggiero G., Tuddenham EG. Coronary thrombosis and the platelet glycoprotein IIIA gene PLA2 polymorphism. Thromb Haemost. 1998. 80:218–9.

16). Goldschmidt-Clermont PJ., Cooke GE., Eaton GM., Binkley PF. PlA2, a variant of GPIIIa implicated in coronary thromboembolic complications. J Am Coll Cardiol. 2000. 36:90–3.

17). Wagner KR., Giles WH., Johnson CJ, et al. Platelet glycoprotein receptor IIIa polymorphism P1A2 and ischemic stroke risk: the stroke prevention in young women study. Stroke. 1998. 29:581–5.
18). Rolf MG., Brearley CA., Mahaut-Smith MP. Platelet shape change evoked by selective activation of P2X1 purinoceptors with alpha, beta-methylene ATP. Thromb Haemost. 2001. 85:303–8.
19). Foster CJ., Prosser DM., Agans JM, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001. 107:1591–8.

20). Woulfe D., Yang J., Brass L. ADP and platelets: the end of the beginning. J Clin Invest. 2001. 107:1503–5.

21). MacKenzie AB., Mahaut-Smith MP., Sage SO. Activation of receptor-operated cation channels via P2X1 not P2T purinoceptors in human platelets. J Biol Chem. 1996. 271:2879–81.

22). Léon C., Hechler B., Freund M, et al. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y(1) receptor-null mice. J Clin Invest. 1999. 104:1731–7.

23). Fontana P., Gaussem P., Aiach M., Fiessinger JN., Emmerich J., Reny JL. P2Y12 H2 haplotype is associated with peripheral arterial disease: a case-control study. Circulation. 2003. 108:2971–3.
24). Huo Y., Ley KF. Role of platelets in the development of atherosclerosis. Trends Cardiovasc Med. 2004. 14:18–22.

25). Fontana P., Dupont A., Grandrille S, et al. Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation. 2003. 108:989–95.
Go to : 
Table 1.
P2Y1 primers
| Primer | Sequence (5'→3') |
|---|---|
| P2Y1-A1622G-F | TCCTCTGAGGAGAAAATCGATGGATGTACCTGGT |
| P2Y1-A1622G-R | AGACACAGCAAAAACAGTCAGTAC |
Table 2.
The P2Y1 polymorphism in patients with ischemic cerebrovascular diseases
Table 3.
The P2Y1 polymorphism in patients with coronary artery diseases




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download