Abstract
Children with Down syndrome (DS) have a higher risk of developing leukemia than do healthy children, and they especially have a higher risk for developing transient myeloproliferative disorder (TMD) or acute megakaryocytic leukemia (AMKL). In recent studies, it has been reported that most of these patients have acquired mutation of the GATA1 gene, which encodes the erythroid/megakaryocytic transcription factor GATA1. GATA1 mutations have not been found in AMKL patients who did not have DS and other hematologic malignancies in DS. Most of the GATA1 mutations in DS-TMD/AMKL are nonsense mutations that are mainly located in exon 2. We observed a nonsense mutation in exon 2 of GATA1 [c.189_190delCA (Tyr63X)] in one case of DS-TMD. The GATA1 mutation has been thought to be an early event in the leukemogenesis of DS-TMD/AMKL and it could be used as a stable molecular marker to assess the treatment response or to monitor for the recurrence of DS-TMD/AMKL.
Go to : 
REFERENCES
1). Zipursky A., Poon A., Doyle J. Leukemia in Down syndrome: a review. Pediatr Hematol Oncol. 1992. 9:139–49.

2). Brink DS. Transient leukemia (transient myeloproliferative disorder, transient abnormal myelopoiesis) of Down syndrome. Adv Anat Pathol. 2006. 13:256–62.

3). Xu G., Nagano M., Kanezaki R, et al. Frequent mutations in the GATA-1 gene in the transient myeloproliferative disorder of Down syndrome. Blood. 2003. 102:2960–8.

4). Wechsler J., Greene M., McDevitt MA, et al. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat Genet. 2002. 32:148–52.

5). Greene ME., Mundschau G., Wechsler J, et al. Mutations in GATA1 in both transient myeloproliferative disorder and acute megakaryoblastic leukemia of Down syndrome. Blood Cells Mol Dis. 2003. 31:351–6.

6). Ahmed M., Sternberg A., Hall G, et al. Natural history of GATA1 mutations in Down syndrome. Blood. 2004. 103:2480–9.

7). Evans T., Felsenfeld G. The erythroid-specific transcription factor Eryf1: a new finger protein. Cell. 1989. 58:877–85.

8). Nichols KE., Crispino JD., Poncz M, et al. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nat Genet. 2000. 24:266–70.

9). Robertson M., De Jong G., Mansvelt E. Prenatal diagnosis of congenital leukemia in a fetus at 25 weeks' gestation with Down syndrome: case report and review of the literature. Ultrasound Obstet Gynecol. 2003. 21:486–9.

10). Stark B., Jeison M., Preudhomme C, et al. Acquired trisomy 21 and distinct clonal evolution in acute megakaryoblastic leukaemia in young monozygotic twins. Br J Haematol. 2002. 118:1082–6.

11). Crispino JD. GATA1 mutations in Down syndrome: implications for biology and diagnosis of children with transient myeloproliferative disorder and acute megakaryoblastic leukemia. Pediatr Blood Cancer. 2005. 44:40–4.

12). Carpenter E., Valverde-Garduno V., Sternberg A, et al. GATA1 mutation and trisomy 21 are required only in haematopoietic cells for development of transient myeloproliferative disorder. Br J Haematol. 2005. 128:548–51.

Go to : 
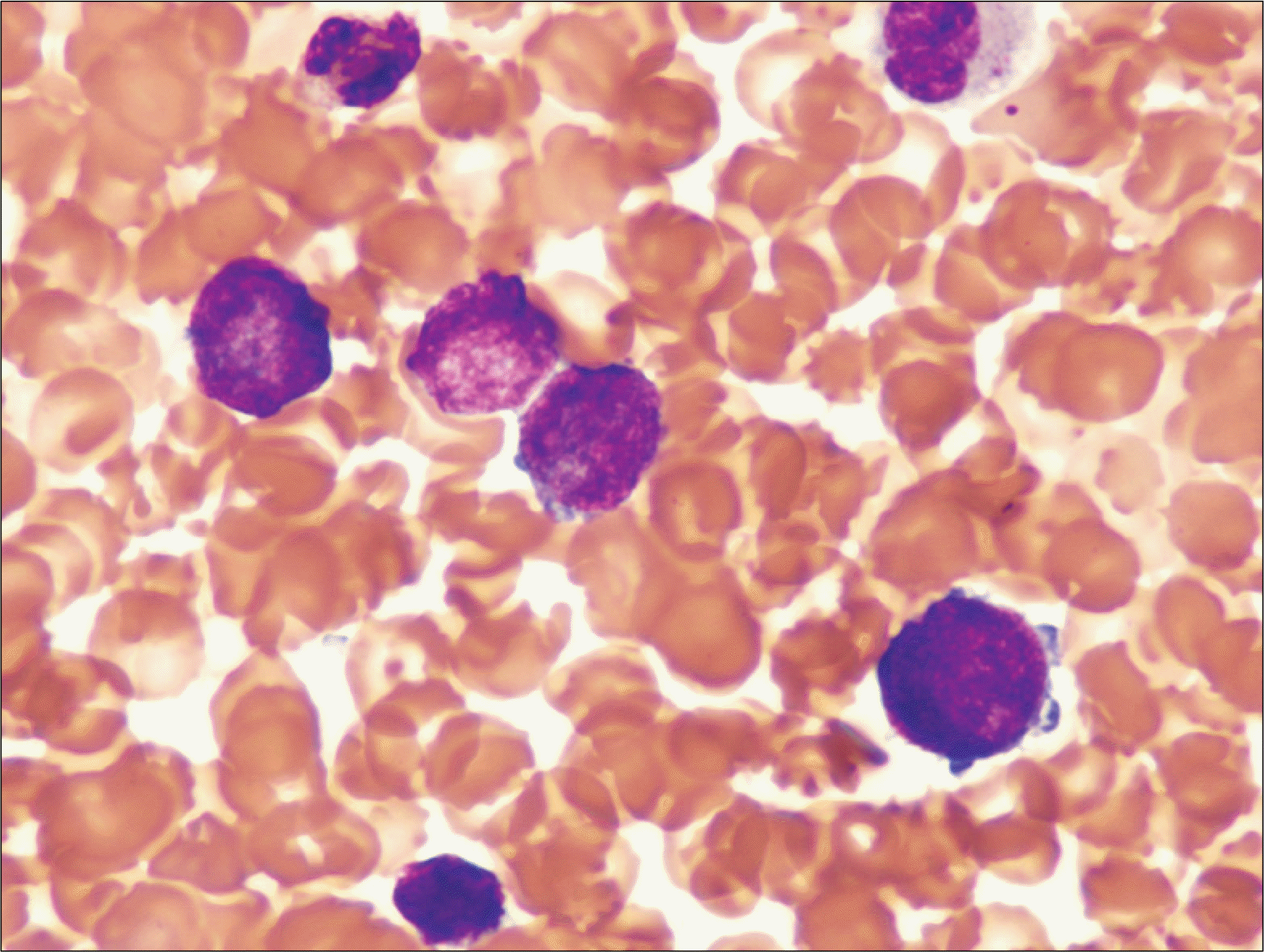 | Fig. 1Bone marrow aspiration smear showing increased immature cells which have less condensed coarse chromatin and cytoplasmic blebbing (Wright-Giemsa stain, ×1,000). |
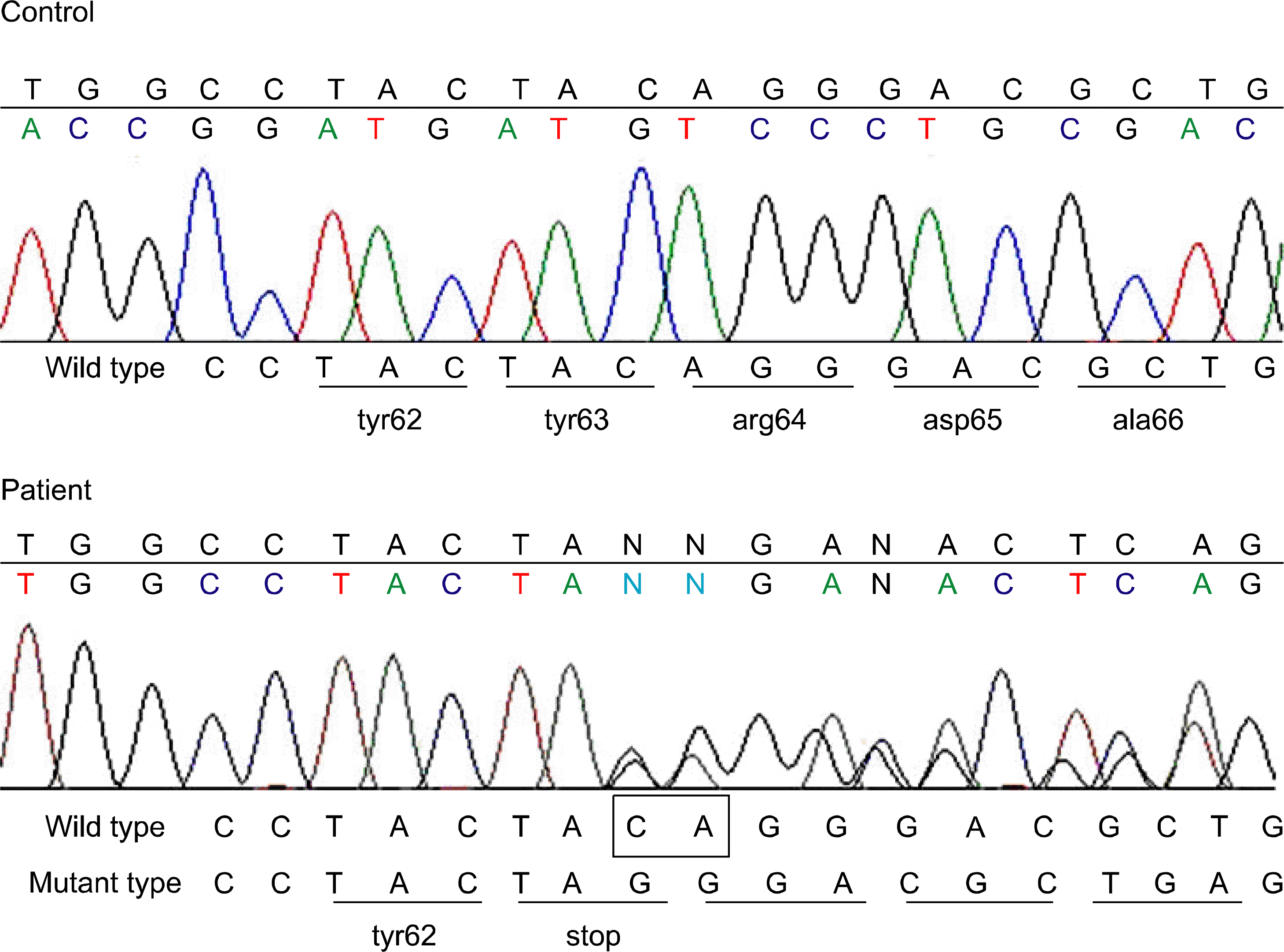 | Fig. 2Direct sequence analysis of exon 2 of GATA1 gene. Wild-type (WT) trace is shown above the patient result for comparison. Note there are two superimposed sequence traces in patient result. Thesu-perimposed traces are wild type and mutant type which has deletion of CA (rectangular) from nucleotides 189 to 190 (c.189_190delCA). This mutation is a nonsense mutation that codon 63 TAC was changed to stop codon TAG. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download