Abstract
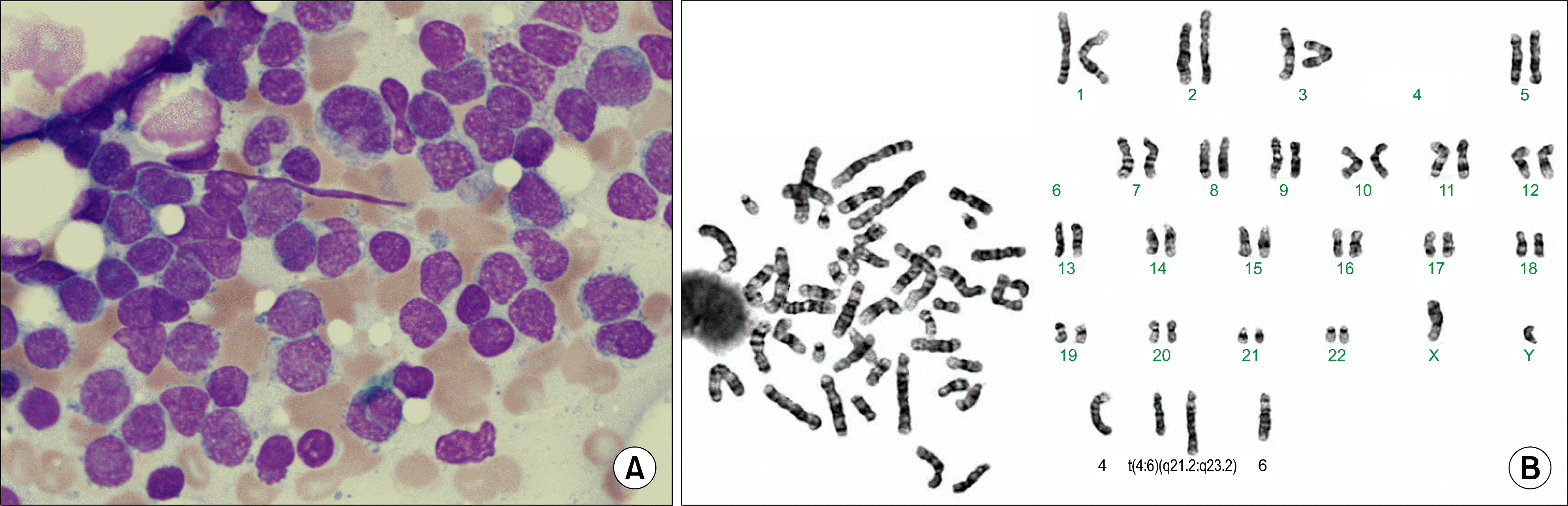
We report here on a case of metachronous second primary non-Hodgkin's lymphoma (NHL) that was diagnosed 6 years after performing subtotal gastrectomy for treating early gastric cancer (EGC). The subtype analysis revealed mantle cell lymphoma (MCL) of the blastic variant with a leukemic presentation, which was composed of mixed small and medium-sized cells. The immunohistochemical staining for cyclin-D1 was positive. The cytogenetic study revealed t(4;6). In Korea, the risk of developing a second primary cancer following gastric cancer was reported to be less than 3.4%, and NHL comprised less than 6.3% of this second primary cancer. Furthermore, MCL represents about 2% of all lymphomas in Korea. To the best of our knowledge, this is the first report of metachronous primary MCL with a leukemic presentation following curative resection of EGC.
REFERENCES
1). Eom BW., Lee HJ., Yoo MW, et al. Synchronous and metachronous cancers in patients with gastric cancer. J Surg Oncol. 2008. 98:106–10.

2). Ryu DD., Um JW., Son GS, et al. Multiple primary malignant tumors in patients with gastric cancer. J Korean Gastric Cancer Assoc. 2003. 3:139–44.

3). Hiyama T., Hanai A., Fujimoto I. Second primary cancer after diagnosis of stomach cancer in Osaka, Japan. Jpn J Cancer Res. 1991. 82:762–70.

4). Hamaloglu E., Topaloglu S., Ozdemir A., Ozenc A. Synchronous and metachronous occurrence of gastric adenocarcinoma and gastric lymphoma: a review of the literature. World J Gastroenterol. 2006. 12:3564–74.

5). Prabhash K., Biswas G., Nair R, et al. Metachronous gastric diffuse large B-cell lymphoma and adenocarcinoma. Indian J Gastroenterol. 2006. 25:261–2.
6). Raderer M., Streubel B., Wöhrer S., Chott A. Metachronous gastric MALT lymphoma and early gastric cancer. Ann Oncol. 2006. 17:724.

7). Banks PM., Chan J., Cleary ML, et al. Mantle cell lymphoma. A proposal for unification of morphologic, immunologic, and molecular data. Am J Surg Pathol. 1992. 16:637–40.

8). Wong KF., Chan JK., So JC., Yu PH. Mantle cell lymphoma in leukemic phase: characterization of its broad cytologic spectrum with emphasis on the importance of distinction from other chronic lymphoproliferative disorders. Cancer. 1999. 86:850–7.
9). Bernard M., Gressin R., Lefrère F, et al. Blastic variant of mantle cell lymphoma: a rare but highly aggressive subtype. Leukemia. 2001. 15:1785–91.

10). Bosch F., López-Guillermo A., Campo E, et al. Mantle cell lymphoma: presenting features, response to therapy, and prognostic factors. Cancer. 1998. 82:567–75.
11). Parrens M., Belaud-Rotureau MA., Fitoussi O, et al. Blastoid and common variants of mantle cell lymphoma exhibit distinct immunophenotypic and interphase FISH features. Histopathology. 2006. 48:353–62.

12). Oinonen R., Franssila K., Teerenhovi L., Lappalainen K., Elonen E. Mantle cell lymphoma: clinical features, treatment and prognosis of 94 patients. Eur J Cancer. 1998. 34:329–36.

13). Matutes E., Parry-Jones N., Brito-Babapulle V, et al. The leukemic presentation of mantle-cell lymphoma: disease features and prognostic factors in 58 patients. Leuk Lymphoma. 2004. 45:2007–15.

14). Witzig TE. Current treatment approaches for mantle-cell lymphoma. J Clin Oncol. 2005. 23:6409–14.

15). Nam MH., Woo HY., Park Q, et al. Mantle cell lymphoma/leukemia in bone marrow: lacking evidence of t(11;14). Korean J Clin Pathol. 2001. 21:437–44.
Fig. 1
The increased FDG uptake in the axial skeleton of whole body is noted. The multifocal increase of FDG uptake in multiple lymph node areas including jugular, paratracheal, internal mammary, subcarinal, hilar, anterior and middle diaphragmatic, portocaval, paraaortic, iliac, inguinal, and mesenteric lymph node areas is noted. The increase of FDG uptake in the liver and spleen is also seen.
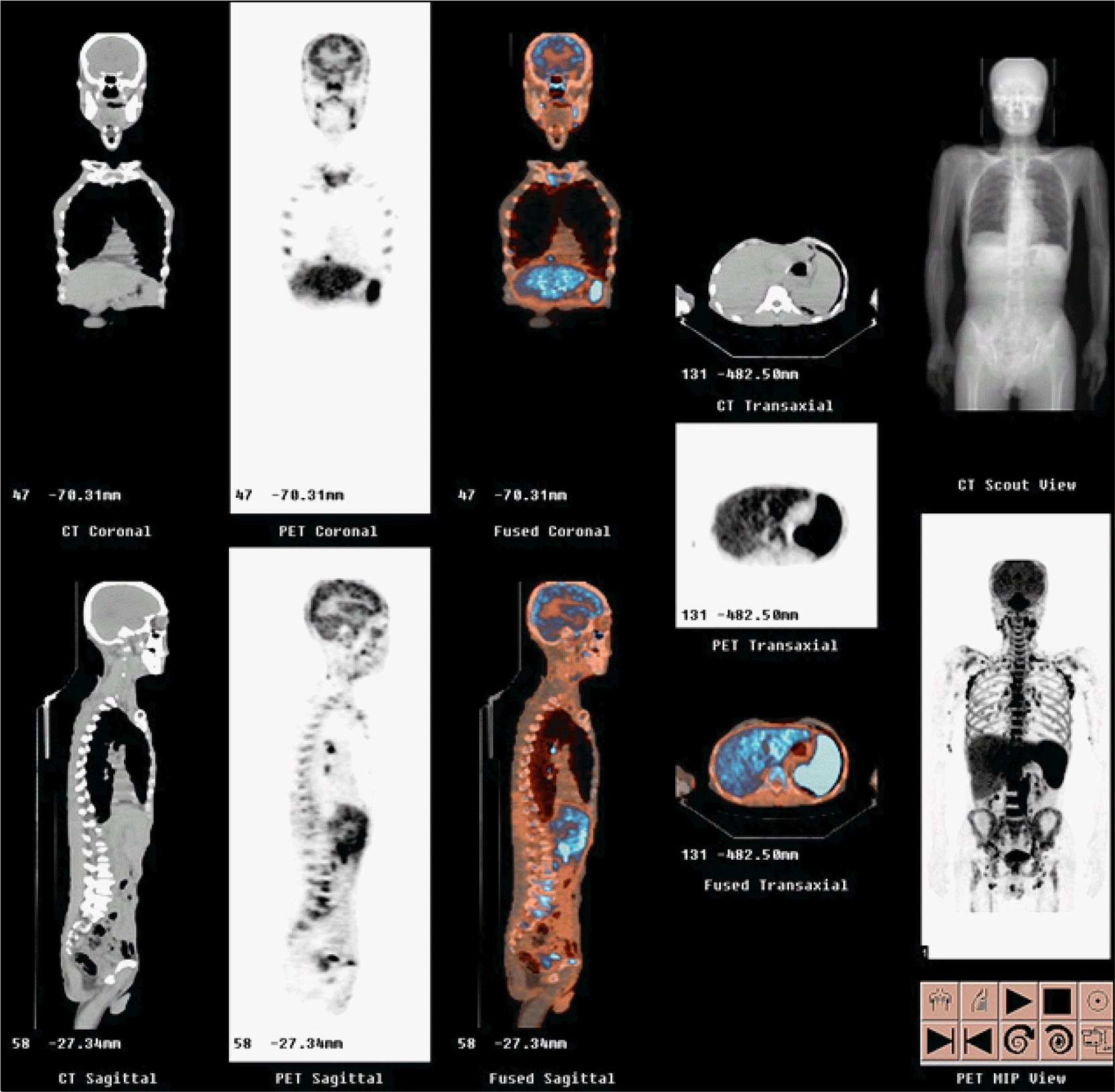
Fig. 2
The microscopic examination of the lymph node from the inguinal area shows diffuse proliferation of small to medium-sized lymphoid cells with irregular nuclear contour, and admixed lymphoid cells with dispersed chromatin resembling lymphoblasts (H-E stain, ×200) (A). The immunohistochemical staining for cyclin-D1 reveals positive result (×400) (B).





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download