Abstract
Background:
The bone marrow (BM) findingd on day 7 of induction chemotherapy is one of major prognostic factors for patients with acute lymphoblastic leukemia (ALL). M3 marrow (blast >25% on BM examination) on day 7 is associated with lower survival rates, compared with the M1 (blast <5%) or M2 (blast 5∼25%) marrow. Herein, we analyzed the effect of augmented post-induction chemotherapy in patients who have high-risk ALL with M3 marrow on the day 7 BM examination.
Methods:
We analyzed the patients who were diagnosed with high-risk ALL and they received modified Children's Cancer Group (CCG)-1882 induction chemotherapy between January 1996 and October 2005 at Seoul National University Children's Hospital. The patients with M1 or M2 marrow on day 7 were treated with modified CCG-1882A/B chemotherapy from consolidation, and the patients with M3 marrow were treated with modified CCG-1882C chemotherapy.
Results:
A total of 44 patients (29 with modified CCG-1882A/B and 15 with modified CCG-1882C) were analyzed. The overall survival (OS) and 5-year event-free survival (EFS) were 86.2% and 81.9%, respectively. The OS of the patients who were treated with the modified CCG-1882A/B protocol (88.9%) was not different from that of the patients who were treated with the modified CCG-1882C protocol (80.0%) (P=0.3256). Also, there was no statistical difference in the 5-year EFS of both groups (85.4% vs 72.7%, respectively, P=0.2117).
Conclusions:
There was no difference of survival between the patients with M1/M2 marrow on the day 7 BM examination and those with M3 marrow after augmented post-induction chemotherapy for the patient with high-risk ALL. We suggest that the poor prognosis of high-risk ALL patients with a poor initial response could be overcome by augmented post-induction chemotherapy.
Go to : 
REFERENCES
1). Nachman JB., Sather HN., Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. New Eng J Med. 1998. 388:1663–71.

2). Reiter A., Schrappe M., Ludwig WD, et al. Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients. Results and conclusions of the multicenter trial ALL-BFM 86. Blood. 1994. 84:3122–33.

3). Gajjar A., Ribeiro R., Hancock ML, et al. Persistence of circulating blasts after 1 week of multiagent chemotherapy confers a poor prognosis in childhood acute lymphoblastic leukemia. Blood. 1995. 86:1292–5.

4). Steinherz PG., Gaynon PS., Breneman JC, et al. Cytoreduction and prognosis in acute lymphoblastic leukemia-the importance of early marrow response: report from the Children's Cancer Group. J Clin Oncol. 1996. 14:389–98.

5). Park DY., Yang CH., Park SH., Lyu CJ., Kim KY. Prognostic significance of circulating blasts after 7 day of multiagent chemotherapy in childhood acute lymphoblastic leukemia. Korean J Pediatr Hematol-Oncol. 1999. 6:293–7.
6). Gaynon PS., Bleyer WA., Steinherz PG, et al. Day 7 marrow response and outcome for children with acute lymphoblastic leukemia and unfavorable presenting features. Med Pediatr Oncol. 1990. 18:273–9.

7). Schultz KR., Massing B., Spinelli JJ., Gaynon PS., Wadsworth L. Importance of the day 7 bone marrow biopsy as a prognostic measure of the outcome in children with acute lymphoblastic leukemia. Med Pediatr Oncol. 1997. 29:16–22.

8). Gaynon PS., Trigg ME., Heerema NA, et al. Children's Cancer Group trials in childhood acute lymphoblastic leukemia: 1983-1995. Leukemia. 2000. 14:2223–33.

9). Nachman J., Sather HN., Cherlow JM, et al. Response of children with high-risk acute lymphoblastic leukemia treated with and without cranial irradiation: a report from the Children's Cancer Group. J Clin Oncol. 1998. 16:920–30.

10). Simone JV. Childhood leukemia-successes and challenges for survivors. N Engl J Med. 2003. 349:627–8.
11). Schultz KR., Pullen DJ., Sather HN, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG). Blood. 2007. 109:926–35.

12). Jacquillat C., Weil M., Gemon MF, et al. Combination therapy in 130 patients with acute lymphoblastic leukemia (Protcol 06 LA 66-Paris). Cancer Res. 1973. 33:3278–84.
13). Tubergen D., Gilchrist GS., O'Brien RT, et al. Improved outcome with delayed intensification for children with acute lymphoblastic leukemia and intermediate presenting features: a Children's Cancer Group phase III trial. J Clin Oncol. 1993. 11:527–37.

14). Nachman J., Sather HN., Gaynon PS., Lukens JN., Wolff L., Trigg ME. Augmented Berlin-Frankfurt-Munster therapy abrogates the adverse prognostic significance of slow early response to induction chemotherapy for children and adolescents with acute lymphoblastic leukemia and unfavorable presenting features: a report from the Children's Cancer Group. J Clin Oncol. 1997. 15:2222–30.

15). Seibel NL., Steinherz PG., Sather HN., Lukens JN., Wolff L., Trigg ME. Early post-induction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2008. 111:2548–55.
16). Kang HJ., Shin HY., Ahn HS. Acute lymphoblastic leukemia in children: past, present and future. Korean J Pediatr. 2007. 50:601–5.

17). Na SO., Shin HY., Ahn HS., Park SK. Study on the treatment of childhood acute lymphoblastic leukemia. J Korean Cancer Assoc. 1992. 24:390–400.
Go to : 
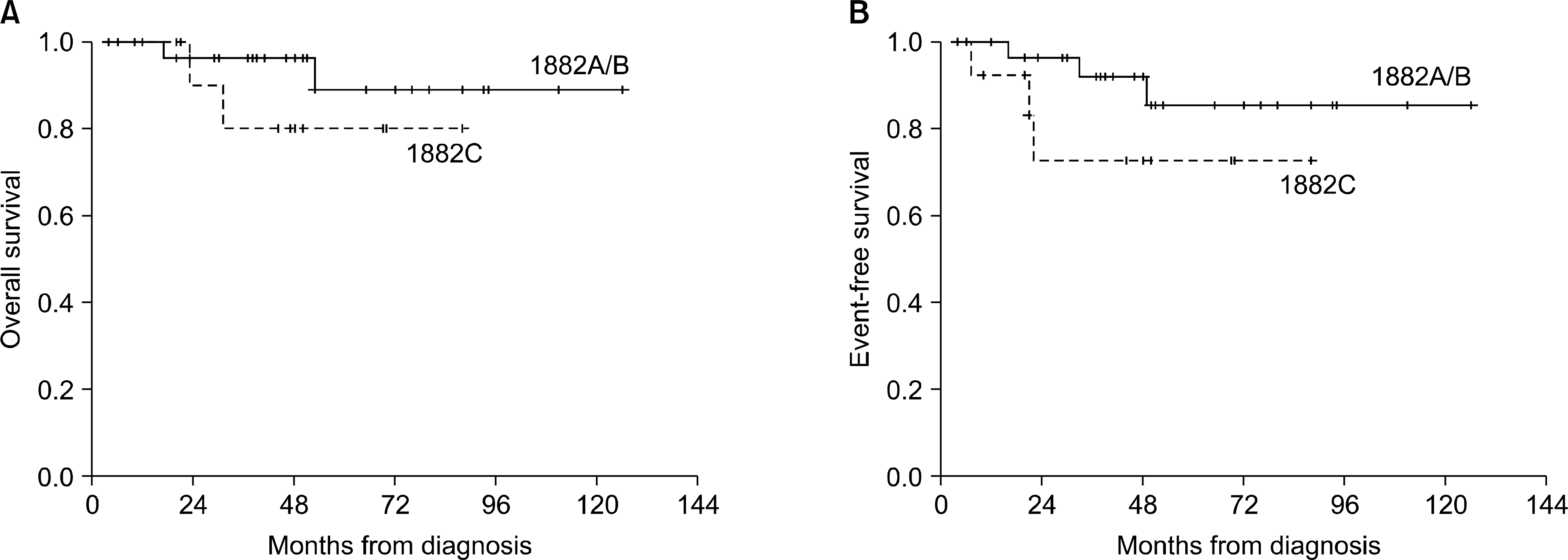 | Fig. 2Overall survival (A) and event-free survival (B) in both groups of post-induction treatment protocols. There was no statistical difference in overall survival (P=0.3256) and event-free survival (P=0.2117) of both groups. |
Table 1.
Original (A) and modified (B) CCG-1882 induction chemotherapy protocol
| (A) | |
| Original CCG-1882 induction chemotherapy protocol | |
| Prednisolone (40mg/m2, oral) | Days 0~27, then tapered over 10 days |
| Vincristine (1.5mg/m2, IV) (max. dose: 2mg) | Days 0, 7, 14, 21 |
| L-asparaginase (6,000IU/m2, IM) | Three times per week, total 9 doses |
| Daunomycin (25mg/m2, IV) | Days 0, 7, 14, 21 |
| Cytarabine (IT, age-adjusted dose∗) | Day 0 |
| Methotrexate (IT, age-adjusted dose†) | Day 14, 28 |
| (B) | |
| Modified CCG-1882 induction chemotherapy protocol | |
| Prednisolone (60mg/m2, oral) | Days 0~27, then tapered over 14 days |
| Vincristine (1.5mg/m2, IV) (max. dose: 2mg) | Days 0, 7, 14, 21 |
| L-asparaginase (6,000IU/m2, IM) | Three times per week, total 9 doses |
| Daunomycin (25mg/m2, IV) (if ANC ≥500/uL and platelet count ≥50,000/uL on day 14 and 21) | Days 0, 7, 14, 21 |
| Cytarabine (IT, age-adjusted dose∗) | Day 0 |
| Methotrexate (IT, age-adjusted dose†) | Day 7, 28 |
Table 2.
Modified CCG-1882A/B and CCG-1882C post-induction chemotherapy protocol
Abbreviations: CCG, Children's Cancer Group; IV, intravenously; SC, subcutaneously; IM, intramuscularly; IT, intrathecally; PO, orally; Triple therapy, cytarabine+hydrocortisone+methotrexate; ANC, absolute neutrophil count. ∗Cranial (1,800cGy) or craniospinal (2,400+600)cGy radiotherapy was done in the consolidation stage.
Table 3.
Characteristics of both treatment groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download