Abstract
Background:
Mesenchymal stem cells (MSCs) may be useful for reducing graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (allo-HSCT). The GVHD and a graft-versus-leukemia (GVL) effect are inversely related. We therefore wanted to determine whether MSCs can preserve the GVL effect following experimental allo-HSCT.
Methods:
After non-myeloablative allogeneic hematopoietic stem cell transplantation (NM-HSCT) using C57BL/6 (H-2b)→B6D2F1 (H-2b/d), some mice received donor lymphocyte infusion (DLI) for induction of GVL effects by virtue of complete chimerism (CC), while the other groups did not receive DLI with persistence of mixed chimerism (MC). All mice were inoculated subcutaneously with P815 tumor cells and were intravenously treated with either donor MSCs or diluents.
Results:
Between the DLI-treated groups with CC, tumor-related deaths and tumor growths were comparable irrespective to the infusion of MSCs. On the contrary, among mice without DLI which showed MC, the administration of MSCs significantly delayed tumor-related deaths compared to those without the administration of MSCs (50-day percent survival, 54.5% vs. 18.1%, P=0.0225). In the MC animals, tumor growth seemed to be more delayed in the mice injected with MSCs than in the controls (P=0.09). Donor MSCs injection was associated with increased donor effector/memory CD62L- T cells in MC but not in CC.
REFERENCES
2). Horowitz MM., Gale RP., Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990. 75:555–62.

3). Friedenstein AJ., Piatetzky-Shapiro II., Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966. 16:381–90.

4). Friedenstein AJ., Chailakhyan RK., Latsinik NV., Panasyuk AF., Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retrans-plantation in vivo. Transplantation. 1974. 17:331–40.
6). Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997. 276:71–4.

7). Pittenger MF., Mackay AM., Beck SC, et al. Multiline-age potential of adult human mesenchymal stemcells. Science. 1999. 284:143–7.
8). Bianchi G., Muraglia A., Daga A., Corte G., Cancedda R., Quarto R. Microenvironment and stem properties of bone marrow-derived mesenchymal cells. Wound Repair Regen. 2001. 9:460–6.

9). Di Nicola M., Carrio-stella C., Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002. 99:3838–43.

10). Le Blanc K., Tammik L., Sundberg B., Haynesworth SE., Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003. 57:11–20.

11). Tse WT., Pendleton JD., Beyer WM., Egalka MC., Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003. 75:389–97.

12). Krampera M., Glennie S., Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003. 101:3722–9.

13). Bartholomew A., Sturgeon C., Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002. 30:42–8.

14). Le Blanc K., Rasmusson I., Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004. 363:1439–41.

15). Ning H., Yang F., Jiang M, et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia. 2008. 22:593–9.

16). Kamate C., Baloul S., Grootenboer S, et al. Inflammation and cancer, the mastocytoma P815 tumor model revisited: triggering of macrophage activation in vivo with pro-tumorigenic consequences. Int J Cancer. 2002. 100:571–9.
17). Spyridonidis A., Bernhardt W., Behringer D, et al. Proliferation and survival of mammary carcinoma cells are influenced by culture conditions used for ex vivo expansion of CD34(+) blood progenitor cells. Blood. 1999. 93:746–55.
18). Min CK., Kim BG., Park G., Cho B., Oh IH. IL-10-transduced bone marrow mesenchymal stem cells can attenuate the severity of acute graft-versus-host disease after experimental allogeneic stem cell transplantation. Bone Marrow Transplant. 2007. 39.

19). Slavin S., Strober S., Fuks Z., Kaplan HS. Induction of specific tissue transplantation tolerance using fractionated total lymphoid irradiation in adult mice: long-term survival of allogeneic bone marrow and skin grafts. J Exp Med. 1977. 146:34–48.

20). Slavin S., Fuks Z., Kaplan HS., Strober S. Transplantation of allogeneic bone marrow without graft-versus-host disease using total lymphoid irradiation. J Exp Med. 19781. 147:963–72.

21). Slavin S., Reitz B., Bieber CP., Kaplan HS., Strober S. Transplantation tolerance in adult rats using total lymphoid irradiation: permanent survival of skin, heart, and marrow allografts. J Exp Med. 1978. 147:700–7.

22). Sykes M., Sachs DH. Mixed allogeneic chimerism as an approach to transplantation tolerance. Immunol Today. 1988. 9:23–7.

23). Sykes M., Sachs DH. Bone marrow transplantation as a means of inducing tolerance. Semin Immunol. 1990. 2:401–17.
24). Weiss L., Reich S., Slavin S. Use of recombinant human interleukin-2 in conjunction with bone marrow transplantation as a model for control of minimal residual disease in malignant hematological disorders: I. treatment of murine leukemia in conjunction with allogeneic bone marrow transplantation and IL-2-activated cell-mediated immunotherapy. Cancer Invest. 1992. 10:19–26.

25). Chen BJ., Cui X., Sempowski GD., Liu C., Chao NJ. Transfer of allogeneic CD62L- memory T cells without graft-versus-host disease. Blood. 2004. 103:1534–41.

26). Le Blanc K., Ringdén O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005. 11:321–34.

27). Le Blanc K., Frassoni F., Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008. 371:1579–86.

28). Kolb HJ., Schmid C., Barrett AJ., Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood. 2004. 103:767–76.

29). Xun CQ., Thompson JS., Jennings CD., Brown SA., Widmer MB. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood. 1994. 83:2360–7.
30). Hill GR., Crawford JM., Cooke KR., Brinson YS., Pan L., Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997. 90:3204–13.

31). Sudres M., Norol F., Trenado A, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. Immunol. 2006. 176:7761–7.

32). Badillo AT., Peranteau WH., Heaton TE., Quinn C., Flake AW. Murine bone marrow derived stromal progenitor cells fail to prevent or treat acute graft-versus-host disease. Br J Haematol. 2008. 141:224–34.

33). Weiden PL., Sullivan KM., Flournoy N., Storb R., Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981. 304:1529–33.
34). Ruggeri L., Capanni M., Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002. 295:2097–100.

35). Kolb HJ., Schattenberg A., Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995. 86:2041–50.
36). Slavin S., Naparstek E., Nagler A., Ackerstein A., Kapelushnik J., Or R. Allogeneic cell therapy for relapsed leukemia after bone marrow transplantation with donor peripheral blood lymphocytes. Exp Hematol. 1995. 23:1553–62.
37). Slavin S., Naparstek E., Nagler A, et al. Allogeneic cell therapy with donor peripheral blood cells and recombinant human interleukin-2 to treat leukemia relapse after allogeneic bone marrow transplantation. Blood. 1996. 87:2195–204.

38). Naparstek E., Or R., Nagler A, et al. T-cell-depleted allogeneic bone marrow transplantation for acute leukaemia using Campath-1 antibodies and posttransplant administration of donor's peripheral blood lymphocytes for prevention of relapse. Br J Haematol. 1995. 89:506–15.

39). Weiss L., Lubin I., Factorowich I, et al. Effective graft-versus-leukemia effects independent of graft-versus-host disease after T cell-depleted allogeneic bone marrow transplantation in a murine model of B cell leukemia/lymphoma. Role of cell therapy and recombinant IL-2. J Immunol. 1994. 153:2562–7.
40). Mobley JL., Rigby SM., Dailey MO. Regulation of adhesion molecule expression by CD8 T cells in vivo. II. Expression of L-selectin (CD62L) by memory cytolytic T cells responding to minor histocompatibility antigens. J Immunol. 1994. 153:5443–52.
41). Ahmadzadeh M., Hussain SF., Farber DL. Heterogeneity of the memory CD4 T cell response: persisting effectors and resting memory T cells. J Immunol. 2001. 166:926–35.

42). Kagamu H., Touhalisky JE., Plautz GE., Krauss JC., Shu S. Isolation based on L-selectin expression of immune effector T cells derived from tumor-draining lymph nodes. Cancer Res. 1996. 56:4338–42.
43). Kagamu H., Shu S. Purification of L-selectin(low) cells promotes the generation of highly potent CD4 antitumor effector T lymphocytes. J Immunol. 1998. 160:3444–52.
44). Nauta AJ., Westerhuis G., Kruisselbrink AB., Lurvink EG., Willemze R., Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a non-myeloablative setting. Blood. 2006. 108:2114–20.

45). Grinnemo KH., Månsson A., Dellgren G, et al. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg. 2004. 127:1293–300.

46). Eliopoulos N., Stagg J., Lejeune L., Pommey S., Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005. 106:4057–65.

47). Pereboeva L., Curiel DT. Cellular vehicles for cancer gene therapy: current status and future potential. BioDrugs. 2004. 18:361–85.
48). Studeny M., Marini FC., Dembinski JL, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004. 96:1593–603.

Fig. 1
Immunophenotype of murine MSCs. Donor MSCs were stained with surface antibodies and analyzed by FACS.
Fig. 2
Responding B6 splenocytes (5×105 cells/well) were incubated for 4 days with mitomicin-treated B6 (Syngeneic) or F1 splenocytes (Allogeneic) in the presence of 1×105 MSCs or diluent alone (Control). Addition of MSCs inhibited T cell proliferative response compared to the controls (∗P=0.005).
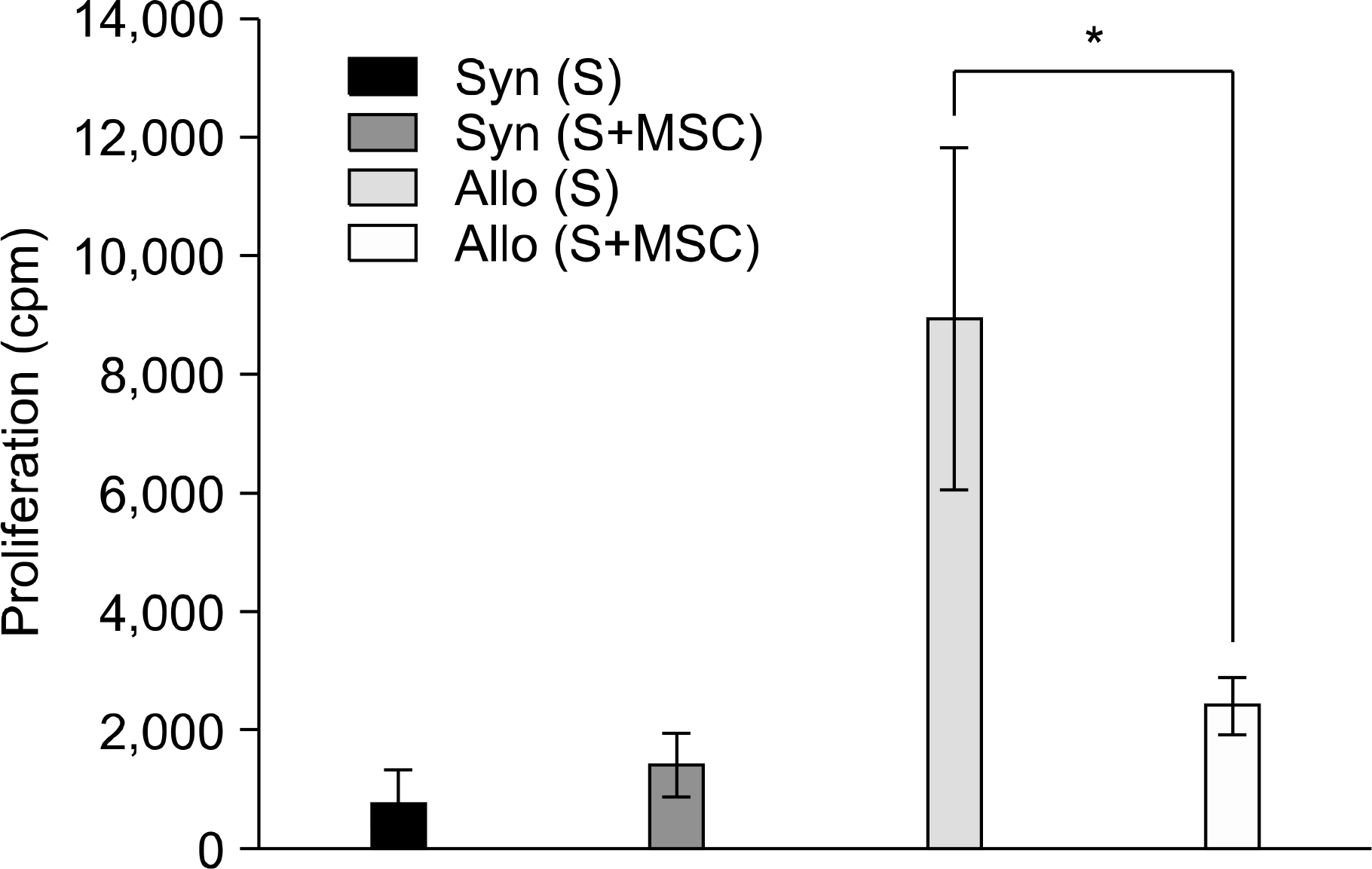
Fig. 3
The percentage of H-2d negative donor cells in peripheral blood following donor lymphocyte infusion.
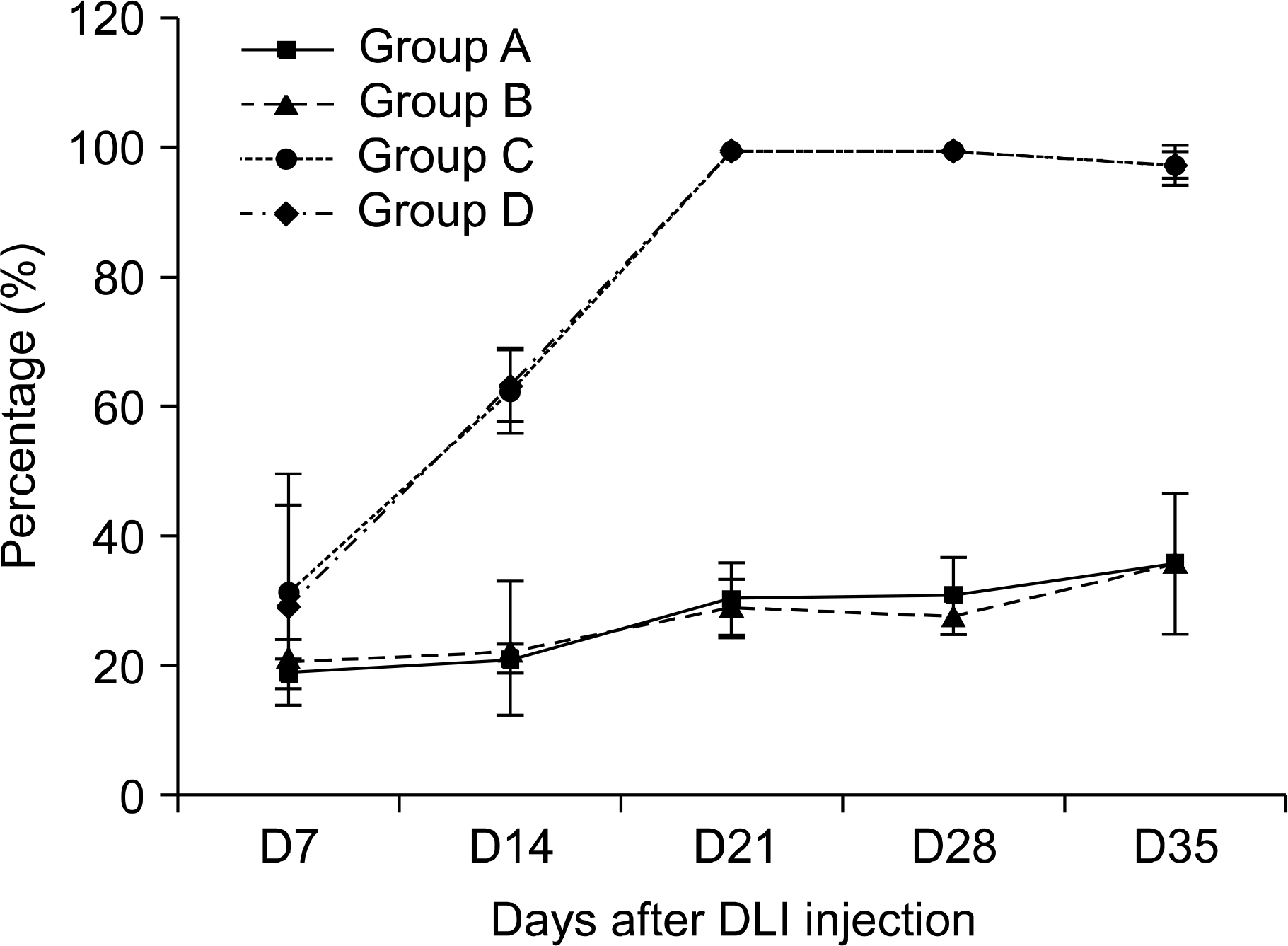
Fig. 4
Effect of MSCs on survival according to the chimeric status. Sublethally irradiated (400cGy) B6D2F1 (H-2b/d) mice were reconstituted with 107 C57BL/6 (H-2b) bone marrow cells. All mice were found to be a state of mixed chimeric (MC). At 14 days after NM-HSCT, group C and D mice received DLI at a dose of 20×106 spleen cells from donor mice for induction of GVL effects by virtue of complete chimerism (CC), while groups A and B did not receive DLI with persistence of MC. At the same, all mice were inoculated subcutaneously with 1×106 P815 cells and then the recipients were intravenously treated on days 15, 18 and 21 with either donor MSCs (group B and D, 5×105/day) or diluents (group A and C). Kalan-Meier survival curves demonstrate a difference between the MC and CC groups (group A vs. B, ∗P=0.0225; group C vs. D, P=0.371).
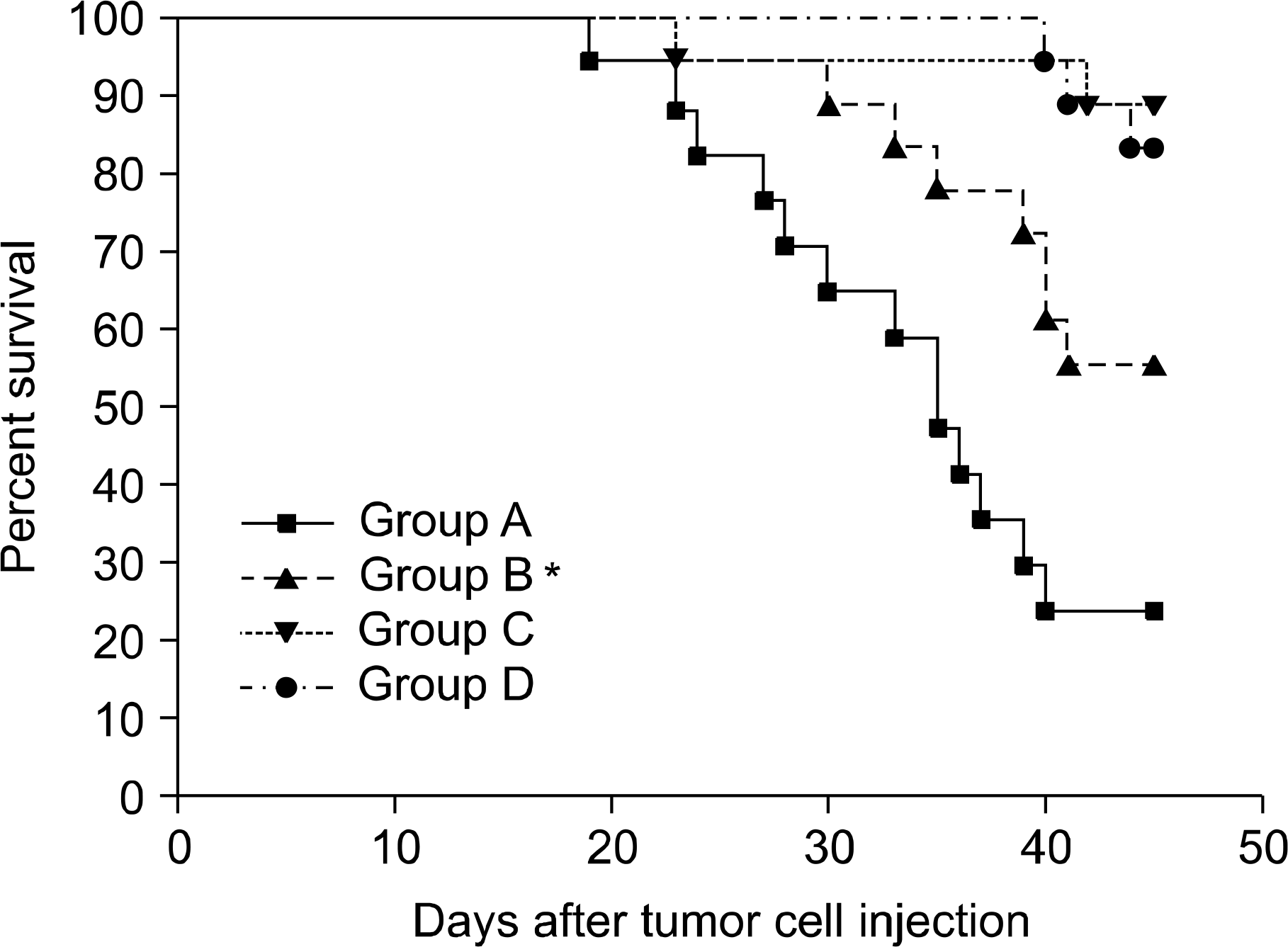
Fig. 5
Flow cytometry analysis of donor and recipient CD62L− memory/effector CD4+ or CD8+ T cells in peripheral blood obtained 21 days after the injection of MSCs. The percentage of circulating donor cells was determined by calculating the number of cells negative for H-2d. Each graph represents one of two similar experiments. (A), Group A; (B), Group B; (C), Group C; (D), Group D.
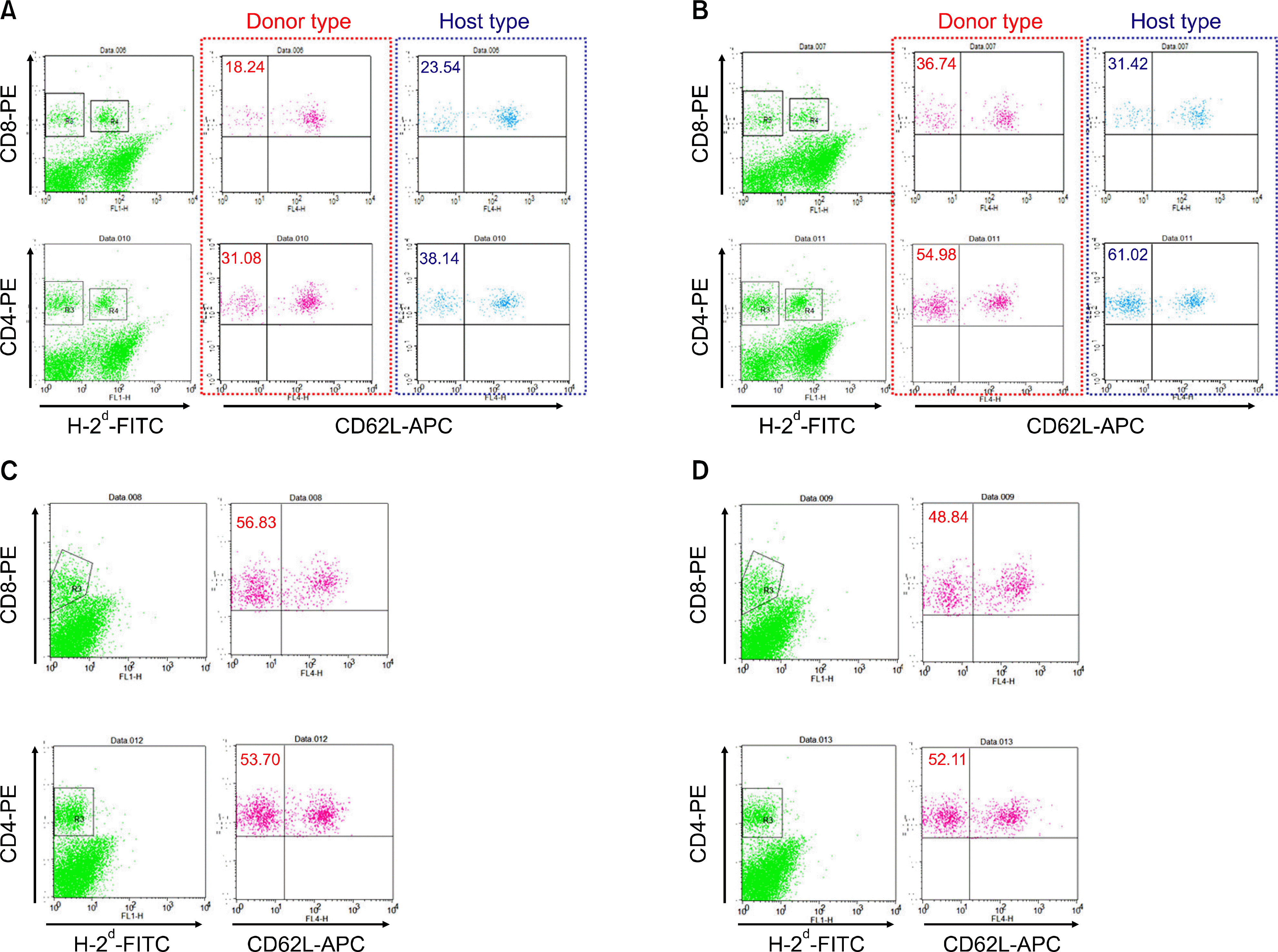
Fig. 6
Tissue distribution of MSCs injected into the recipients with MC (group B). In addition to the tumor tissue, immunohistochemical analysis of lungs, liver, and spleen sections was used to visualize the prelabeled MSCs by their PKH+ red fluorescence (magnification: ×100). Mice in Group A did not receive the prelabeled MSCs.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download