Abstract
Microtype BCR/ABL rearrangement is extremely rare and has been known to be associated with neutrophilic chronic myeloid leukemia (N-CML). However, there is more to be understood regarding this phenotype. We report a case of atypical CML that exhibited micro BCR/ABL rearrangement with predominant thrombocytosis. Our patient showed thrombocytosis (1,464×109/L) and megakaryocytosis in the peripheral blood and bone marrow. However, neither leukocytosis nor neutrophilia was observed (white blood cells (WBC), 5.02×109/L neutrophils, 45%). Bone marrow aspirate revealed increased cellularity: 12% basophils, 6% eosinophils, and 9% blasts. The 46,XX,t(9;22)(q34;q11.2),i(17q) chromosome complement was observed in 4 of 20 metaphase cells, and standard BCR/ABL fusion signals were observed in 10% of interphase cells on fluorescence in situ-hybridization (FISH) analysis. Reverse transcriptase-polymerase chain reaction (RT-PCR) was used to acquire the BCR/ABL fusion transcript, the identity of which was confirmed via sequence analysis. Hematologic remission was achieved 2 months after imatinib therapy initiation, and molecular remission was achieved 2 months after hematologic remission. The patient is currently undergoing regular follow-up visits and is in good health. However, further long-term follow up is warranted. The incorporation of imatinib into therapeutic strategies may be further established through the study of more cases of micro BCR/ABL.
Go to : 
REFERENCES
1). Saglio G., Guerrasio A., Rosso C, et al. New type of Bcr/Abl junction in Philadelphia chromosome-positive chronic myelogenous leukemia. Blood. 1990. 76:1819–24.

2). Pane F., Frigeri F., Sindona M, et al. Neutrophilic-chronic myeloid leukemia: a distinct disease with a specific molecular marker (BCR/ABL with C3/A2 junction). Blood. 1996. 88:2410–4.
3). Wada H., Mizutani S., Nishimura J, et al. Establishment and molecular characterization of a novel leukemic cell line with Philadelphia chromosome expressing p230 BCR/ABL fusion protein. Cancer Res. 1995. 55:3192–6.
4). Rotoli B., Pane F., Salvatore F., Saligo G. The e19a2 bcr/abl breakpoint in acute lymphoblastic leukemia. Br J Haematol. 2000. 110:493–6.
5). Inokuchi K., Dan K., Takatori M, et al. Myeloprolifer-ative disease in transgenic mice expressing P230 Bcr/Abl: longer disease latency, thrombocytosis, and mild leukocytosis. Blood. 2003. 102:320–3.

6). Li S., Ilaria RL Jr., Million RP., Daley GQ., Van Etten RA. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J Exp Med. 1999. 189:1399–412.

7). Lee JJ., Kim HJ., Kim YJ, et al. Imatinib induces a cytogenetic response in blast crisis or interferon failure chronic myeloid leukemia patients with e19a2 BCR-ABL transcripts. Leukemia. 2004. 18:1539–40.

8). Li X., Yang J., Chen X, et al. A report of early cytogenetic response to imatinib in two patients with chronic myeloid leukemia at accelerated phase and carrying the e19a2 BCR-ABL transcript. Cancer Genet Cytogenet. 2007. 176:166–8.

9). Andrikovics H., Nahajevszky S., Szilvási A, et al. First and second line imatinib treatment in chronic myelogenous leukemia patients expressing rare e1a2 or e19a2 BCR-ABL transcripts. Hematol Oncol. 2007. 25:143–7.

10). Kojima K., Yasukawa M., Ishimaru F, et al. Additional translocation (8;21)(q22;q22) in a patient with Philadelphia-positive chronic myelogenous leukaemia in the blastic phase. Br J Haematol. 1999. 106:720–2.

11). Polák J., Koza V., Cetkovský P., Haskovec C. An acce-lerrated-phase CML patient with e19a2 junction of BCR/ABL gene - the first case of transplanted CML with micro bcr. Bone Marrow Transplant. 2000. 25:1109–10.

12). Ohsaka A., Hoshino S., Kobayashi M., Kudo H., Kawaguchi R. Blast crisis of Philadelphia chromosome-positive chronic myeloid leukaemia carrying micro-bcr breakpoint (e19a2 and e191a). Br J Hae-matol. 2002. 118:251–4.

13). Haskovec C., Ponzetto C., Polák J, et al. P230 BCR/ABL protein may be associated with an acute leukaemia phenotype. Br J Haematol. 1998. 103:1104–8.
14). Bernasconi P., Calatroni S., Boni M., Cavigliano PM., Pagnucco G., Bernasconi C. P230 does not always predict a mild clinical course in myeloid malignancies: e19a2 bcr/abl fusion transcript with additional chromosome abnormalities in a patient with acute monoblastic leukemia (M5a). Hematologica. 2001. 86:320–1.
15). Mondal BC., Majumdar S., Dasgupta UB., Chaudhuri U., Chakrabarti P., Bhattacharyya S. E19a2 BCR-ABL fusion transcript in typical chronic myeloid leukemia: a report of two cases. J Clin Pathol. 2006. 59:1102–3.
Go to : 
 | Fig. 1Bone marrow aspiration smear (A, wright stain, ×1,000) and bone marrow biopsy (B, H&E stain, ×200). (A) The bone marrow aspirate shows increased blasts (9%), basophils (12%), and eosinophils (6%). (B) Biopsy shows increased cellularity and proliferation of hypolobulated megakaryocytes. |
 | Fig. 2FISH analysis of BCR/ABL fusion. The interphase cell have one separate green (BCR), one separate red (ABL) and two fusion signals consistent with the presence of a BCR/ABL fusion. |
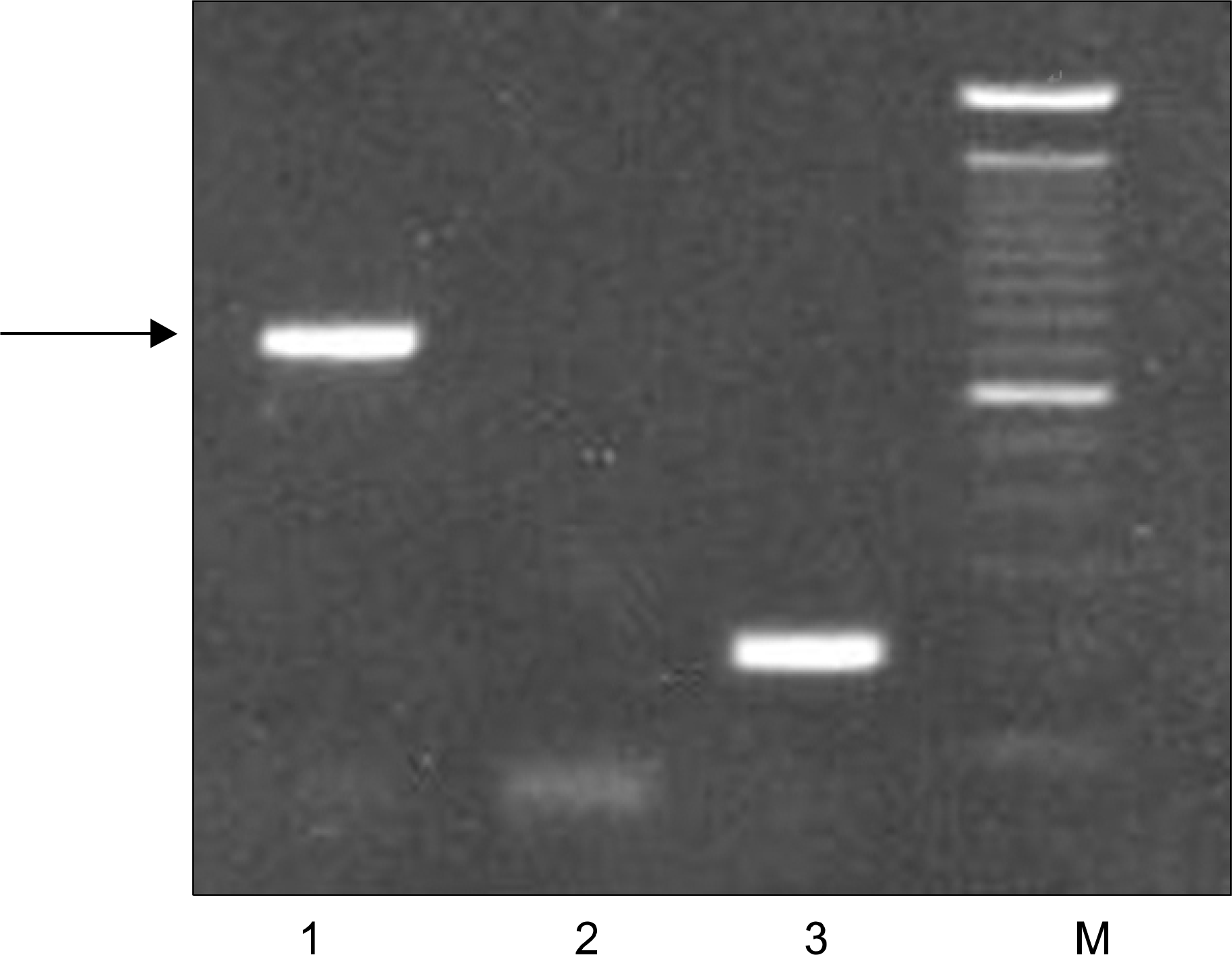 | Fig. 3RT-PCR for the BCR/ABL gene. Lane 1, micro BCR/ABL rearrangement cells of the patient; Lane 2, negative control; Lane 3, positive control with major BCR/ABL rearrangement; M, size marker. |
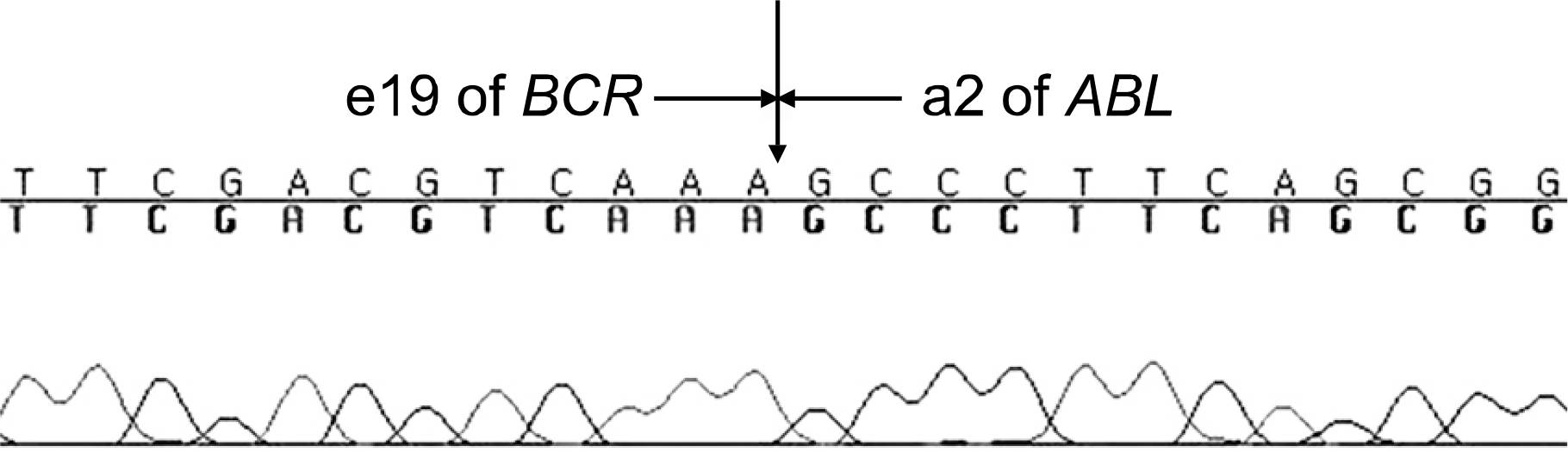 | Fig. 4Sequence analysis. The vertical arrow marks the junction between BCR exon 19 and ABL exon 2. |
Table 1.
Clinical and hematologic chracteristics of the 21 cases with e19a2 junction transcripts reported in the literature




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download