Abstract
Background:
Treatment of T-cell lymphoblastic lymphoma (T-LBL) with CHOP or CHOP-like chemotherapy has resulted in poor long-term outcomes. High-dose chemotherapy followed by ASCT has been applied for this dreaded disease. However, the efficacy is still controversial. T-LBL is considered the nodal/extranodal presentation of acute lymphoblastic leukemia. Favorable results with VPDL chemotherapy have been reported in the setting of adult lymphoblastic leukemia. We, therefore, treated T-LBL patients with modified VPDL chemotherapy and compared the outcomes with those achieved using upfront ASCT.
Methods:
We retrospectively reviewed the outcomes of 24 T-LBL patients treated either with upfront ASCT (n=11) or VPDL chemotherapy without ASCT (n=13) between January 1996 and October 2005.
Results:
The median follow-up duration for surviving patients was 17 months (range, 5∼109 months). The two-year event-free survival (EFS) rates were 83.1% in the VPDL group and 27.3% in the upfront ASCT group (P=0.008). The two-year overall survival (OS) rates were 83.9% in the VPDL group and 27.3% in the upfront ASCT group (P=0.006).
Go to : 
REFERENCES
1). A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997. 89:3909–18.
2). Jaffe ES., Harris NL., Stein H., Vardiman JW. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. World Health Organization classification of tumours. IARC, Lyon. 2001.
3). Voakes JB., Jones SE., McKelvey EM. The chemotherapy of lymphoblastic lymphoma. Blood. 1981. 57:186–8.

4). Morel P., Lepage E., Brice P, et al. Prognosis and treatment of lymphoblastic lymphoma in adults: a report on 80 patients. J Clin Oncol. 1992. 10:1078–85.

5). Bouabdallah R., Xerri L., Bardou VJ, et al. Role of induction chemotherapy and bone marrow transplantation in adult lymphoblastic lymphoma: a report on 62 patients from a single center. Ann Oncol. 1998. 9:619–25.

6). Kaiser U., Uebelacker I., Havemann K. Non-Hodg-kin's lymphoma protocols in the treatment of patients with Burkitt's lymphoma and lymphoblastic lymphoma: a report on 58 patients. Leuk Lymphoma. 1999. 36:101–8.

7). Verdonck LF., Dekker AW., de Gast GC., Lokhorst HM., Nieuwenhuis HK. Autologous bone marrow transplantation for adult poor-risk lymphoblastic lymphoma in first remission. J Clin Oncol. 1992. 10:644–6.

8). Santini G., Coser P., Chisesi T, et al. Autologous bone marrow transplantation for advanced stage adult lymphoblastic lymphoma in first complete remission. A pilot study of the non-Hodgkin's Lymphoma Co-operative Study Group (NHLCSG). Bone Marrow Transplant. 1989. 4:399–404.
9). Song K., Barnett MJ., Gascoyne RD, et al. Primary therapy for adults with T-cell lymphoblastic lymphoma with hematopoietic stem-cell transplantation results in favorable outcomes. Ann Oncol. 2007. 18:535–40.

10). Sweetenham JW., Santini G., Qian W, et al. High-dose therapy and autologous stem-cell transplantation versus conventional-dose consolidation/maintenance therapy as postremission therapy for adult patients with lymphoblastic lymphoma: results of a randomized trial of the European Group for Blood and Marrow Transplantation and the United Kingdom Lymphoma Group. J Clin Oncol. 2001. 19:2927–36.

11). Linker CA., Levitt LJ., O'Donnell M., Forman SJ., Ries CA. Treatment of adult acute lymphoblastic leukemia with intensive cyclical chemotherapy: a follow-up report. Blood. 1991. 78:2814–22.

12). Carbone PP., Kaplan HS., Musshoff K., Smithers DW., Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971. 31:1860–1.
13). A predictive model for aggressive non-Hodgkin's lymphoma. The international non-Hodgkin's lymphoma prognostic factors project. N Engl J Med. 1993. 329:987–94.
14). Cheson BD., Horning SJ., Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999. 17:1244.
15). Soslow RA., Baergen RN., Warnke RA. B-lineage lymphoblastic lymphoma is a clinicopathologic entity distinct from other histologically similar aggressive lymphomas with blastic morphology. Cancer. 1999. 85:2648–54.

16). Thomas DA., O'Brien S., Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004. 104:1624–30.

17). Bernasconi C., Brusamolino E., Lazzarino M., Morra E., Pagnucco G., Orlandi E. Lymphoblastic lymphoma in adult patients: clinicopathological features and response to intensive multiagent chemotherapy analogous to that used in acute lymphoblastic leukemia. Ann Oncol. 1990. 1:141–6.

18). Zinzani PL., Bendandi M., Visani G, et al. Adult lymphoblastic lymphoma: clinical features and prognostic factors in 53 patients. Leuk Lymphoma. 1996. 23:577–82.

19). Coleman CN., Picozzi VJ Jr., Cox RS, et al. Treatment of lymphoblastic lymphoma in adults. J Clin Oncol. 1986. 4:1628–37.

20). Slater DE., Mertelsmann R., Koziner B, et al. Lymphoblastic lymphoma in adults. J Clin Oncol. 1986. 4:57–67.

21). Colgan JP., Andersen J., Habermann TM, et al. Long-term follow-up of a CHOP-based regimen with maintenance therapy and central nervous system prophylaxis in lymphoblastic non-Hodgkin's lymphoma. Leuk Lymphoma. 1994. 15:291–6.
22). Chen YC., Ho CL., Kao WY., Hwang JM., Sheu LF., Chao TY. Adult lymphoblastic lymphoma in Taiwan: an analysis of treatment results of 26 patients. Ann Hematol. 2001. 80:647–52.
Go to : 
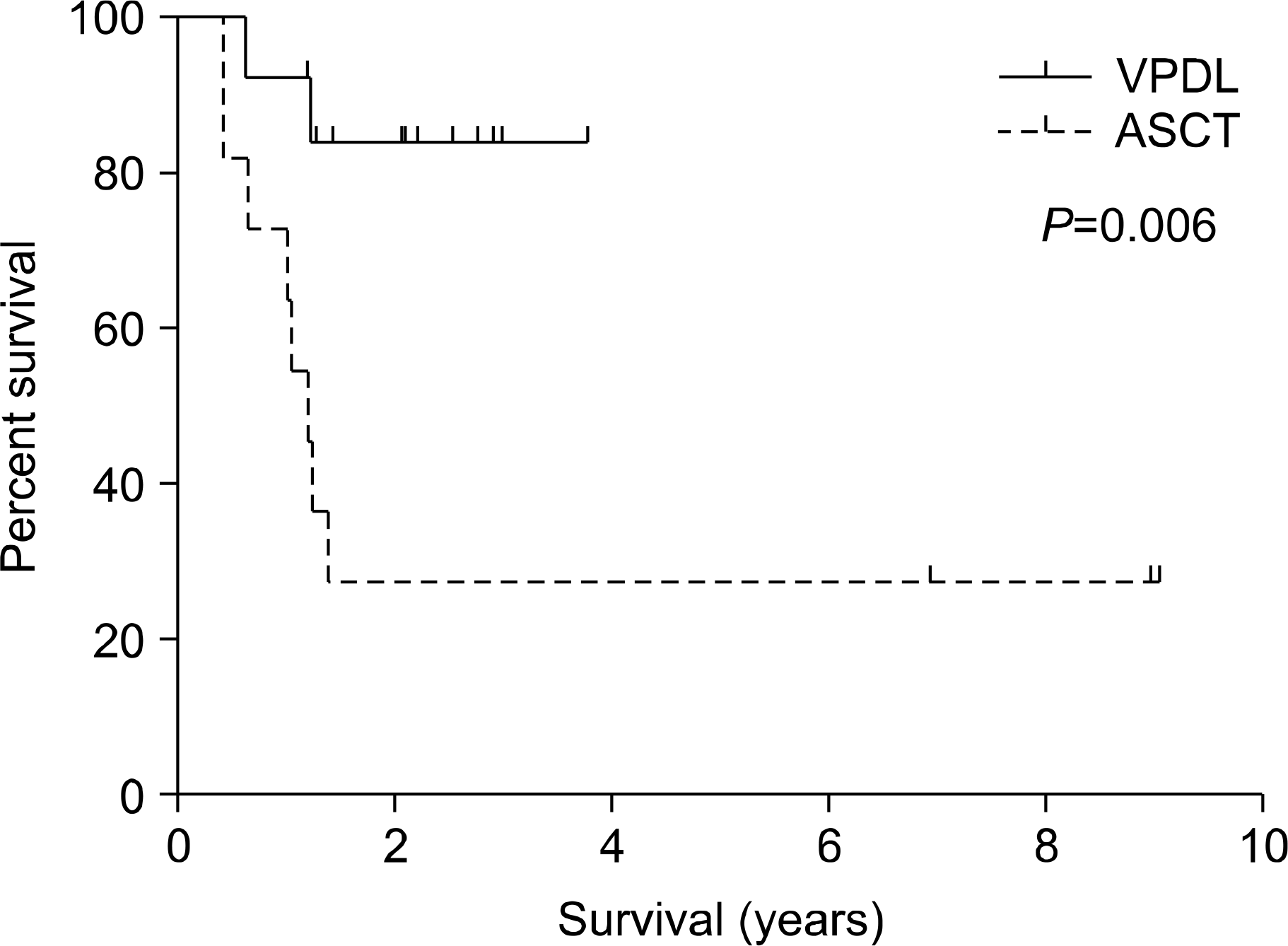 | Fig. 1Probability of overall survival in 13 patients treated with VPDL chemotherapy (solid line) and 11 patients with ASCT (dashed line). |
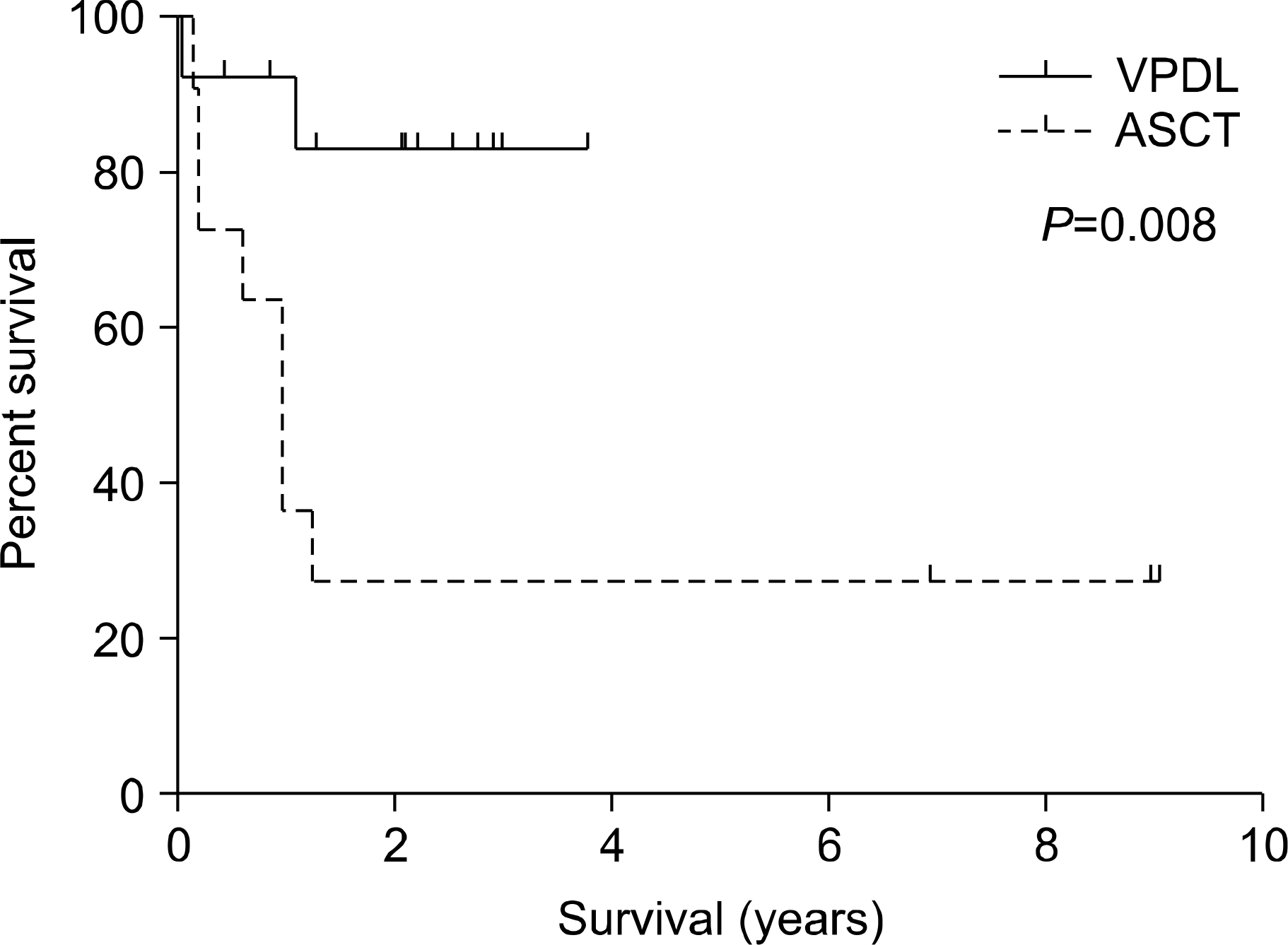 | Fig. 2Probability of event free survival in 13 patients treated with VPDL chemotherapy (solid line) and 11 patients with ASCT (dashed line). |
Table 1.
Prior chemotherapy regimens in the ASCT arm
∗Patients who underwent VPDL induction treatment (See Table 2). Abbreviations: CODOX-M, cyclophosphamide, doxorubicin, vincristine, cytarabine and methotrexate (iv and intrathecal);CVPD, cyclophosphamide, vincristine, dexamethasone, and doxorubicin; DHAP, cytarabine, cisplatin, and dexamethasone;ESHAP, etoposide methylprednisolone, cytarabine, and cisplatin; IVAC, ifosfamide, etoposide, cytarabin, and methotrexate (intrathecal); Vanderbilt, cyclophosphamide, etoposide, vincristine, bleomycin, methotrexate, and prednisolone;VPDL, vincristine, prednisolone, daunorubicin, and L-asparaginase.
Table 2.
Drugs used in the VPDL protocol
Table 3.
Characteristics of all patients at presentation
Table 4.
Patient outcomes after VPDL or ASCT
| VPDL (n=13) | ASCT (n=11) | P-value | |
|---|---|---|---|
| Response | 0.695 | ||
| CR | 9 (69.2%) | 6 (54.5%) | |
| PR | 2 (15.4%) | 2 (18.2%) | |
| PD | 2 (15.4%) | 2 (18.2%) | |
| NA | - | 1 (9.1%)∗ | |
| Status | 0.006 | ||
| Alive | 10 (76.9%) | 3 (27.3%) | |
| Dead | 2 (15.4%) | 8 (72.7%) | |
| Lost to follow up | 1 (7.7%) | - | |
| Cause of death | 0.625 | ||
| Disease progression | 1 (7.7%) | 3 (37.5%) | |
| Infection | 1 (7.7%) | 3 (37.5%) | |
| Other | - | 2 (25.0%)† | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download