Abstract
Background:
The question as to whether stromal cell-derived factor-1 (SDF-1) stimulated myeloma cell growth has been controversial. We explored the possibility that SDF-1 may function as an autocrine growth factor of myeloma cells.
Methods:
CD138+ primary bone marrow myeloma cells and myeloma cell lines (RPMI8226, U266, and ARH77) were used. Chemotaxis in response to SDF-1 of the cells was analyzed using TranswellsTM. Cell proliferation was measured by a colorimetric assay. SDF-1 mRNA expression was analyzed by RT-PCR (reverse-transcription-polymerase chain reaction). SDF-1 and interleukin-6 (IL-6) receptor expression as well as signaling molecule phosphorylation levels, were examined using Western blot analysis. Concentrations of SDF-1 in the cell culture supernatants were measured by ELISA assay.
Results:
SDF-1 alone had no discernible effect on the proliferation of CD138+ primary myeloma cells or myeloma cell lines. In contrast, SDF-1 significantly enhanced IL-6-induced proliferation of these cells. SDF-1 up-regulated the expression of IL-6 receptor and enhanced phosphorylation of AKT in an additive manner with IL-6. Co-culture of the myeloma cells with umbilical vein endothelial cells over-expressing the SDF-1 gene revealed that SDF-1 played an important role in not only the migration of the cells underneath the stromal cells but also the proliferation of the cells in contact with stromal cells. All the myeloma cell lines expressed SDF-1 mRNA, and SDF-1 was detected in the culture supernatants of the cells. The G protein-coupled receptor inhibitor, pertussis toxin, inhibited the proliferation of these cells in suspension cultures.
REFERENCES
1). Peled A., Petit I., Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999. 283:845–8.

2). Aiuti A., Webb IJ., Bleul C., Springer T., Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997. 185:111–20.

3). Dürig J., Schmücker U., Dührsen U. Differential expression of chemokine receptors in B cell malignancies. Leukemia. 2001. 15:752–6.

4). Sanz-Rodriguez F., Hidalgo A., Teixidó J. Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-mediated multiple myeloma cell adhesion to CS-1/fibronectin and VCAM-1. Blood. 2001. 97:346–51.
5). Möller C., Strömberg T., Juremalm M., Nilsson K., Nilsson G. Expression and function of chemokine receptors in human multiple myeloma. Leukemia. 2003. 17:203–10.

6). Parmo-Cabañas M., Bartolomé RA., Wright N., Hidalgo A., Drager AM., Teixidó J. Integrin alpha4beta1 involvement in stromal cell-derived factor-1alpha-promoted myeloma cell transendothelial migration and adhesion: role of cAMP and the actin cytoskeleton in adhesion. Exp Cell Res. 2004. 294:571–80.
7). Zannettino AC., Farrugia AN., Kortesidis A, et al. Elevated serum levels of stromal-derived factor-1al-pha are associated with increased osteoclast activity and osteolytic bone disease in multiple myeloma patients. Cancer Res. 2005. 65:1700–9.
8). Pellegrino A., Ria R., Di Pietro G, et al. Bone marrow endothelial cells in multiple myeloma secrete CXC-chemokines that mediate interactions with plasma cells. Br J Haematol. 2005. 129:248–56.

9). Guleng B., Tateishi K., Ohta M, et al. Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates in vivo tumor growth by inhibiting angiogenesis in a vascular endothelial growth factor-independent manner. Cancer Res. 2005. 65:5864–71.

10). Hideshima T., Chauhan D., Hayashi T, et al. The biological sequelae of stromal cell-derived factor-1alpha in multiple myeloma. Mol Cancer Ther. 2002. 1:539–44.
11). Jaffe EA., Nachman RL., Becker CG., Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973. 52:2745–56.

12). Ramalingam R., Rafii S., Worgall S., Brough DE., Crystal RG. E1(−)E4(+) adenoviral gene transfer vectors function as a "pro-life" signal to promote survival of primary human endothelial cells. Blood. 1999. 93:2936–44.

13). Aikawa S., Hatta Y., Tanaka M, et al. Requirement of soluble factors produced by bone marrow stromal cells on the growth of novel established human myeloma cell line. Int J Oncol. 2003. 22:631–7.
14). Derksen PW., de Gorter DJ., Meijer HP, et al. The hepatocyte growth factor/Met pathway controls proliferation and apoptosis in multiple myeloma. Leukemia. 2003. 17:764–74.

15). Qiang YW., Yao L., Tosato G., Rudikoff S. Insulin-like growth factor I induces migration and invasion of human multiple myeloma cells. Blood. 2004. 103:301–8.

16). Caligaris-Cappio F., Gregoretti MG., Merico F, et al. Bone marrow microenvironment and the progression of multiple myeloma. Leuk Lymphoma. 1992. 8:15–22.

17). Nefedova Y., Landowski TH., Dalton WS. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia. 2003. 17:1175–82.

18). Uchiyama H., Barut BA., Mohrbacher AF., Chauhan D., Anderson KC. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood. 1993. 82:3712–20.

19). Le Gouill S., Podar K., Amiot M, et al. VEGF induces Mcl-1 up-regulation and protects multiple myeloma cells against apoptosis. Blood. 2004. 104:2886–92.

20). Chatterjee M., Hönemann D., Lentzsch S, et al. In the presence of bone marrow stromal cells human multiple myeloma cells become independent of the IL-6/gp130/STAT3 pathway. Blood. 2002. 100:3311–8.

21). Michigami T., Shimizu N., Williams PJ, et al. Cell-cell contact between marrow stromal cells and myeloma cells via VCAM-1 and alpha(4)beta(1)-integrin enhances production of osteoclast-stimulating activity. Blood. 2000. 96:1953–60.
22). Neben S., Anklesaria P., Greenberger J., Mauch P. Quantitation of murine hematopoietic stem cells in vitro by limiting dilution analysis of cobblestone area formation on a clonal stromal cell line. Exp Hematol. 1993. 21:438–43.
23). Breems DA., Blokland EA., Neben S., Ploemacher RE. Frequency analysis of human primitive haematopoietic stem cell subsets using a cobblestone area forming cell assay. Leukemia. 1994. 8:1095–104.
Fig. 1
Myeloma cells express functional CXCR4. (A) Flow cytometric analysis of the expression of CXCR4 on RPMI8226 cells, U266 cells, ARH77 cells, and CD138+ primary myeloma cells. ARH77 cells express CXCR4 minimally on the cell surface; however, analysis after permeabilization reveals CXCR4 in the cytoplasm of the majority of the cells. (B) Chemoattraction of myeloma cells induced by SDF-1 (100 ng/mL) over 4 hr measured using 24-well Transwells with 5-μm pores. Values represent the mean migration index (±SD) obtained from triplicate experiments for cell lines and those obtained from the experiments using five different specimens for CD138+ cells. ∗P<0.05 compared with control.
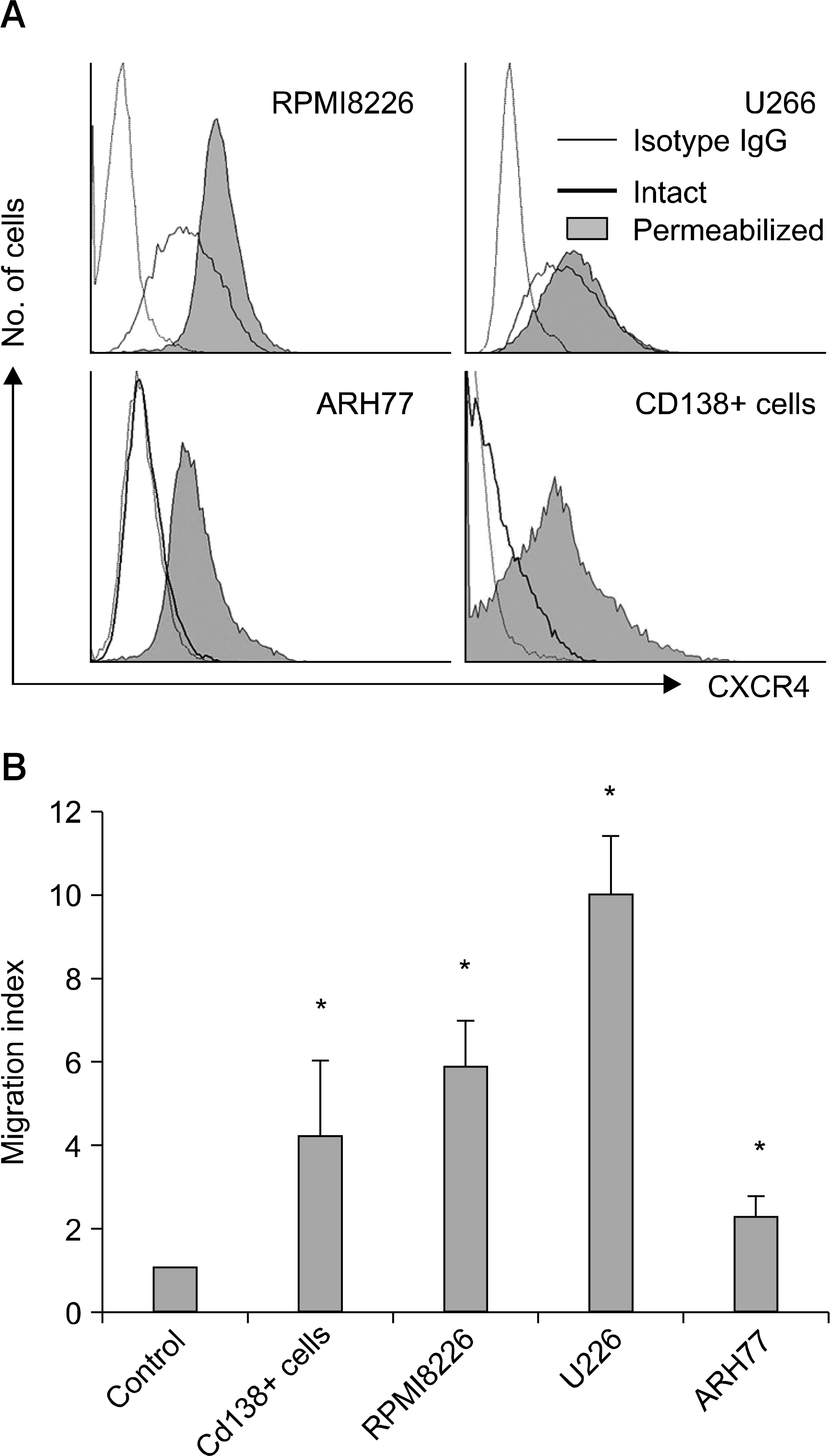
Fig. 2
SDF-1 enhances the proliferation of myeloma cells in concert with IL-6. Cells were incubated in X-VIVO medium for 3 days in the absence or presence of SDF-1 (100ng/mL), IL-6 (20ng/mL), or both. (A) Proliferation of RPMI8226 cells. Data are the means and SD of the proliferation index of the cells from three independent experiments. (B) Proliferation of CD138+ primary myeloma cells. Data are the means and SD of the proliferation index of the cells from three independent experiments using three different specimens. ∗P<0.05.
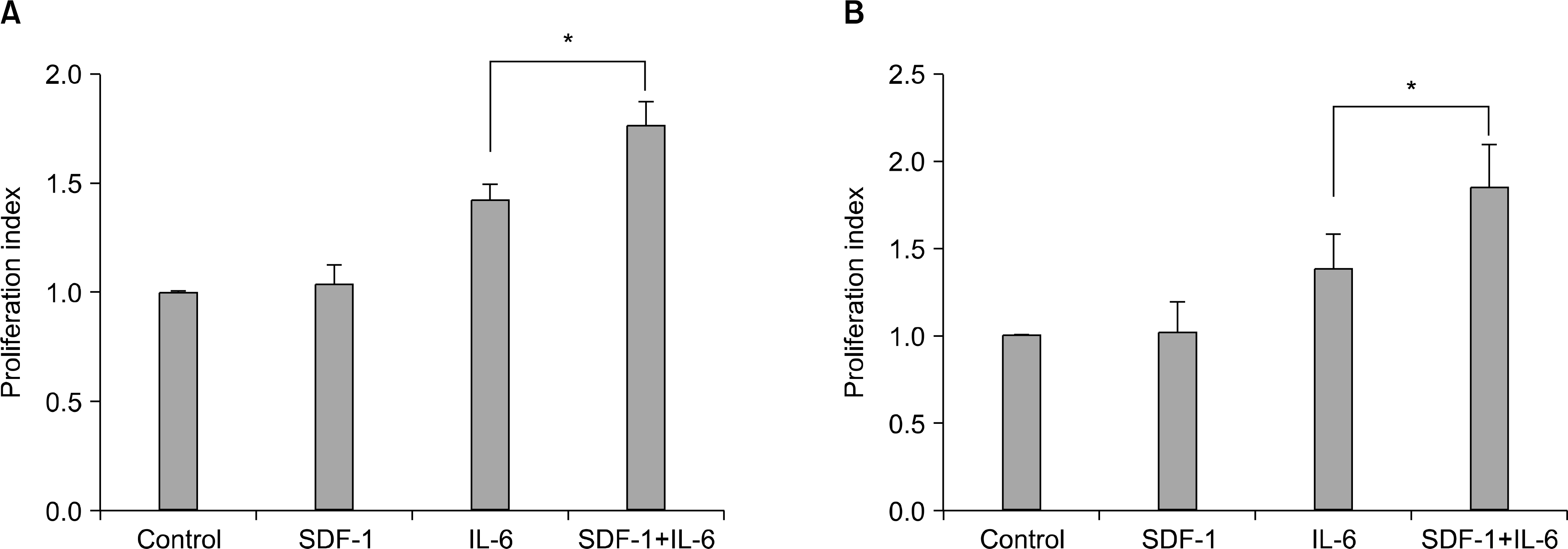
Fig. 3
Effects of IL-6 on the expression of CXCR4 and effect of SDF-1 on the expression of IL-6 receptor in myeloma cells. (A) IL-6 (20ng/mL) does not alter CXCR4 expression on the cell surface, as analyzed by flow cytometry. (B) In contrast, SDF-1 treatment (100ng/mL) for 24 hr up-regulates IL-6 receptor in RPMI8226 and U266 cells, as assessed by Western blot analysis. A representative result of two independent experiments is shown.
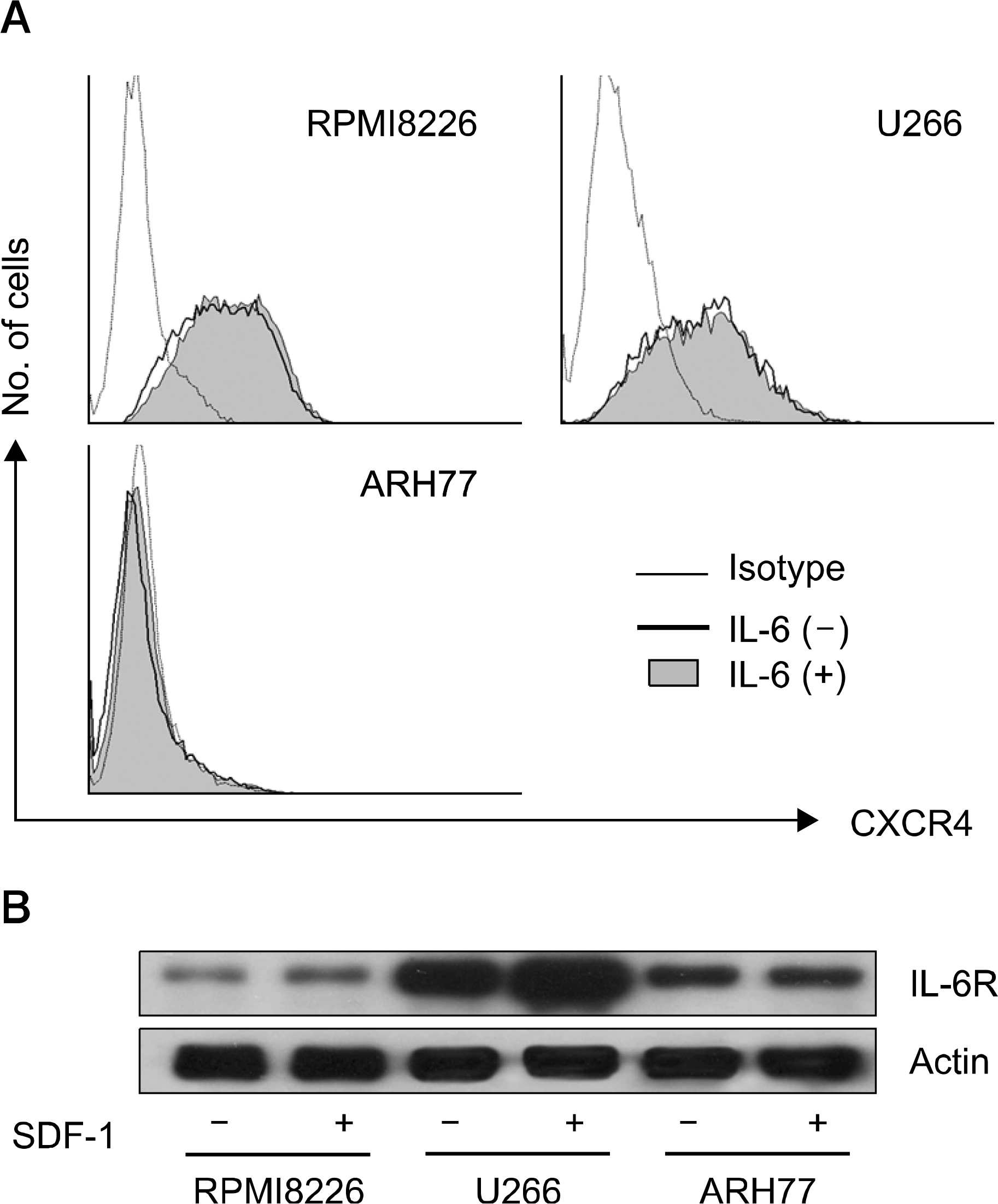
Fig. 4
Effects of SDF-1 and IL-6 on phosphorylation of AKT, ERK, and Stat3 in RPMI8226 cells. (A) Both SDF-1 (100ng/mL) and IL-6 (20ng/mL) increase the phsphorylation of AKT, and the combination enhances the phosphorylation of AKT compared to each cytokine. (B) SDF-1 modestly increases the phosphoryoation of ERK, and IL-6 strongly increases the phosphorylation of ERK. However, the combination does not enhance the phosphorylation. (C) SDF-1 does not cause the phosphorylation of Stat3 and does not enhance the phosphorylation induced by IL-6. A representative result of two independent experiments is shown.
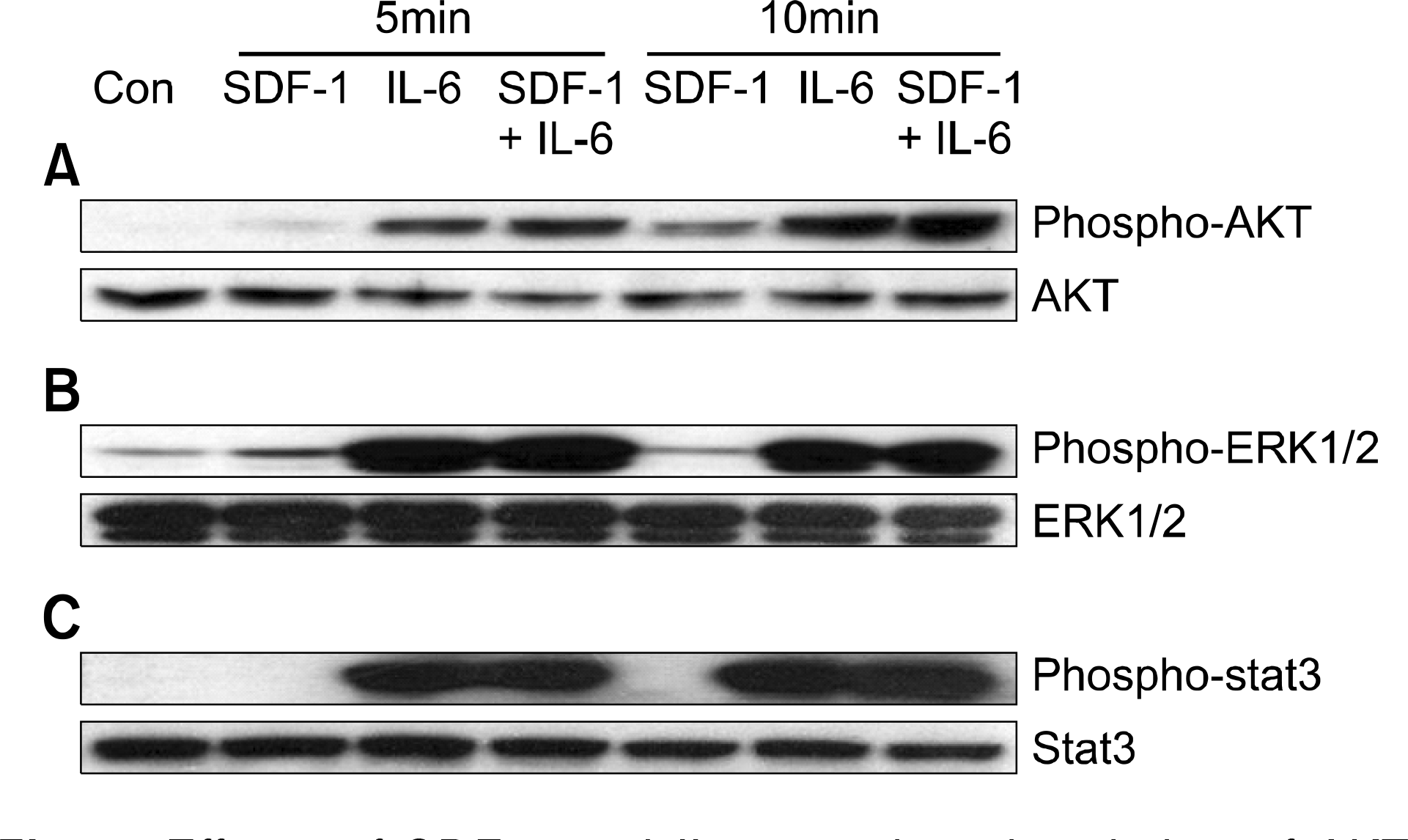
Fig. 5
SDF-1 induces both the migration of myeloma cells beneath the stromal cells and the formation of cobblestone areas. (A) RPMI8226 cells were added onto MS-5 cell feeder layers in X-VIVO medium. The migration of the cells beneath the feeder layer was identified under an inverted microscope 24 hr later. (B) CD138+ primary myeloma cells were cultured in X-VIVO medium on established HUVEC layers, which had been transduced with the LacZ or SDF-1 gene using adenoviral vectors. A representative result of the 2-week culture with HUVECs transduced with the SDF-1 gene is shown. Many cobblestone areas are observed. (C) CD138+ primary myeloma cells (1×104 cells) were cultured on HUVEC layers transduced with the LacZ (AdeLacZ) or SDF-1 gene (AdeSDF-1). As indicated, the cells were pretreated with pertussis toxin (PTX; 200ng/mL) before co-culture. After 2 weeks, cobblestone areas were scored under a phase-contrast inverted microscope. Data are the means and SD of the numbers of cobblestone areas from three independent experiments using different specimens. ∗P<0.05.
Fig. 6
Myeloma cells express SDF-1 mRNA and produce SDF-1. (A) The expression of the mRNA for SDF-1 and CXCR4 in myeloma cells, as analyzed by RT-PCR. (B) Western blot analysis for SDF-1 and CXCR4 in the cells. (C) SDF-1 concentrations in 3-day culture supernatants of the cells, as measured by ELISA.
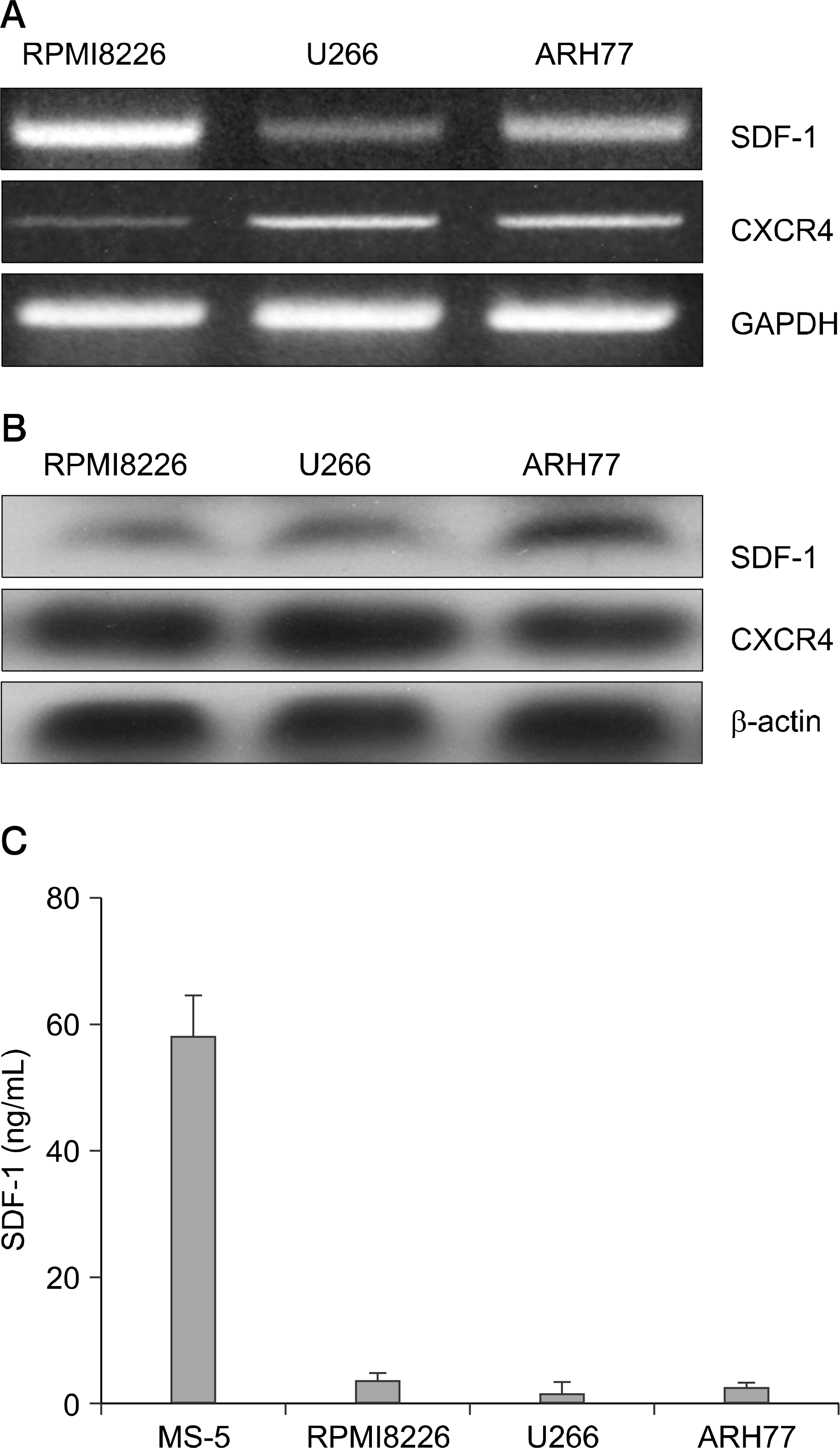
Fig. 7
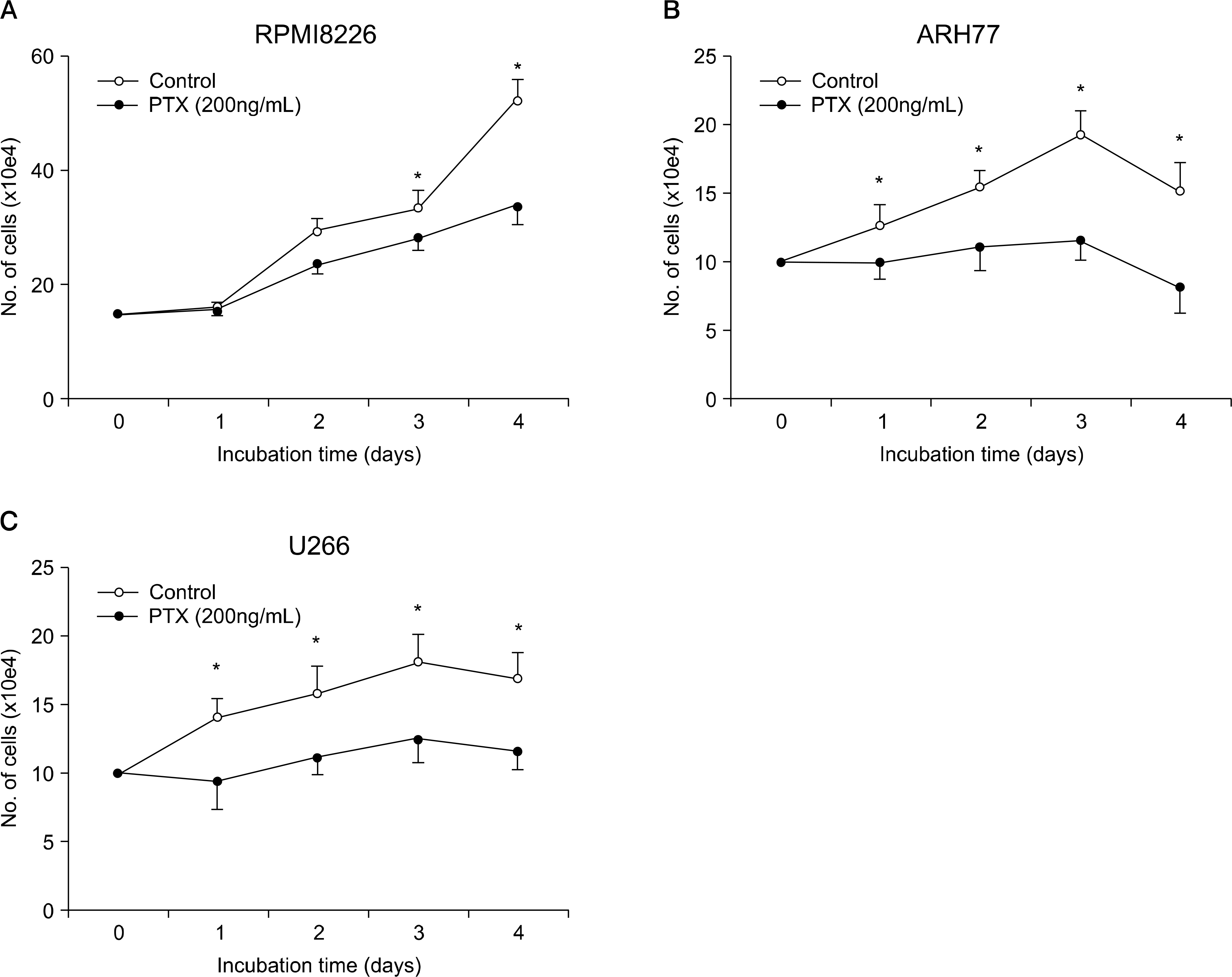
Pertussis toxin (PTX) inhibits the proliferation of myeloma cells. (A) RPMI8226 cells. (B) ARH77 cells. (C) U266 cells. The cells were cultured in X-VIVO medium for up to 4 days in the presence or absence of PTX (200ng/mL). Data are the means and SD of the numbers of cells from triplicate experiments. ∗P <0.05.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download