1). Jaffe ES., Harris NL., Stein H., Vardiman JW. World Health Organization classification of tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press. 2001. 61–73.
2). Vardiman JW., Harris NL., Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002. 100:2292–302.

3). Greenberg PL., Baer MR., Bennett JM, et al. NCCN practice guideline in oncology: Myelodysplastic syndromes-v.1.2007. (Accessed March 2, 2007, at. http://www.nccn.org/professionals/physician_gls/PDF/mds.pdf.
4). Valent P., Horny HP., Bennett JM, et al. Definitions and standards in the diagnosis and treatment of the myelodysplastic syndromes: consensus statements and report from a working conference. Leuk Res. 2007. 31:727–36.

5). Vardiman JW. Hematopathological concepts and controversies in the diagnosis and classification of myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program. 2006. 199–204.

6). Kussick SJ., Fromm JR., Rossini A, et al. Four-color flow cytometry shows strong concordance with bone marrow morphology and cytogenetics in the evaluation for myelodysplasia. Am J Clin Pathol. 2005. 124:170–81.

7). Kong SY., Nam MH., Woo HY, et al. Assessment of the diagnostic utility of methylmalonic acid in megaloblastic anemia due to vitamin B12 deficiency. Korean J Lab Med. 2002. 22:145–52.
8). Santini V., Kantarjian HM., Issa JP. Changes in DNA methylation in neoplasia: pathophysiology and therapeutic implications. Ann Intern Med. 2001. 134:573–86.

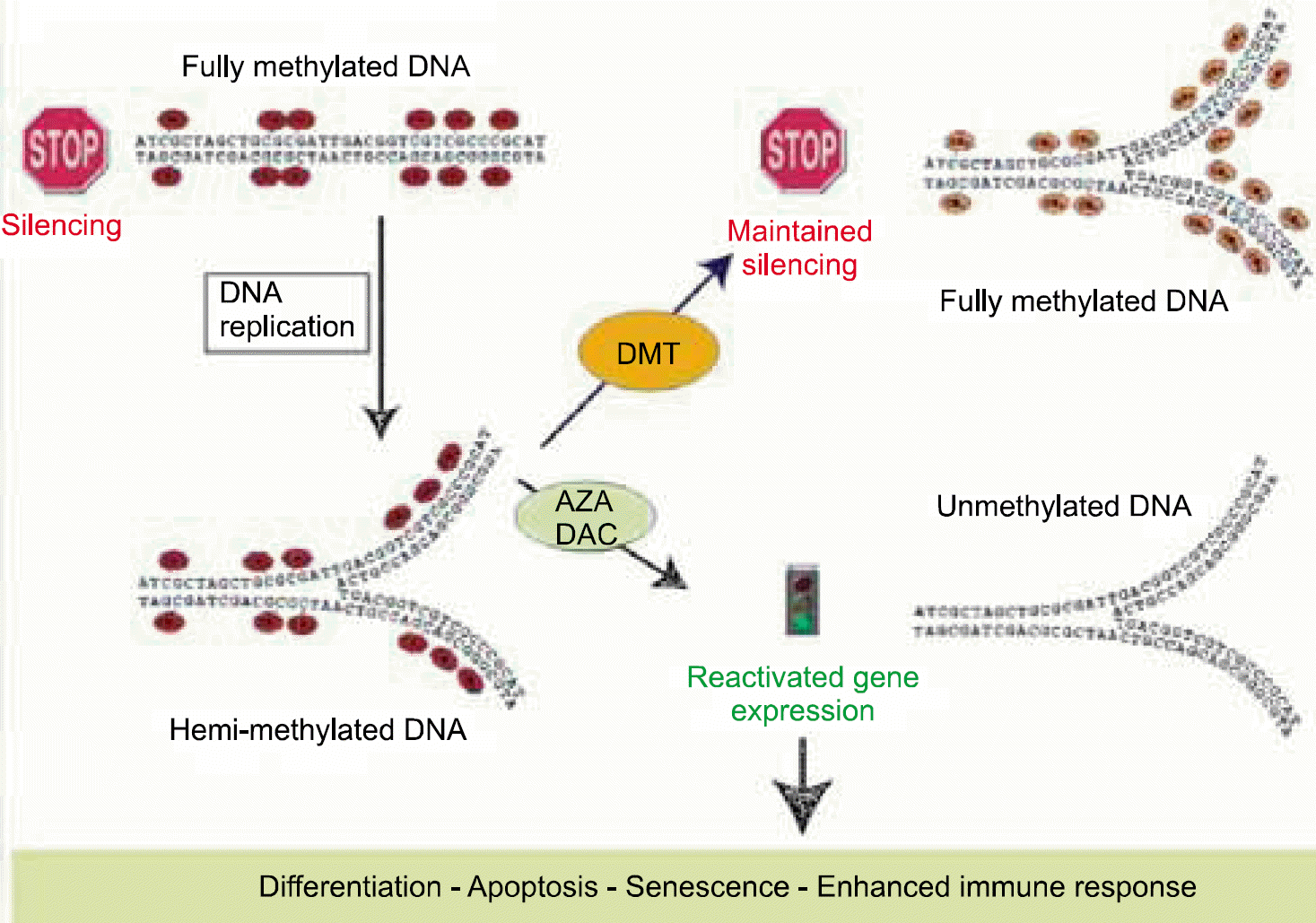
9). Lübbert M. Gene silencing of the p15/INK4B cell-cycle inhibitor by hypermethylation: an early or later epigenetic alteration in myelodysplastic syndromes? Leukemia. 2003. 17:1762–4.

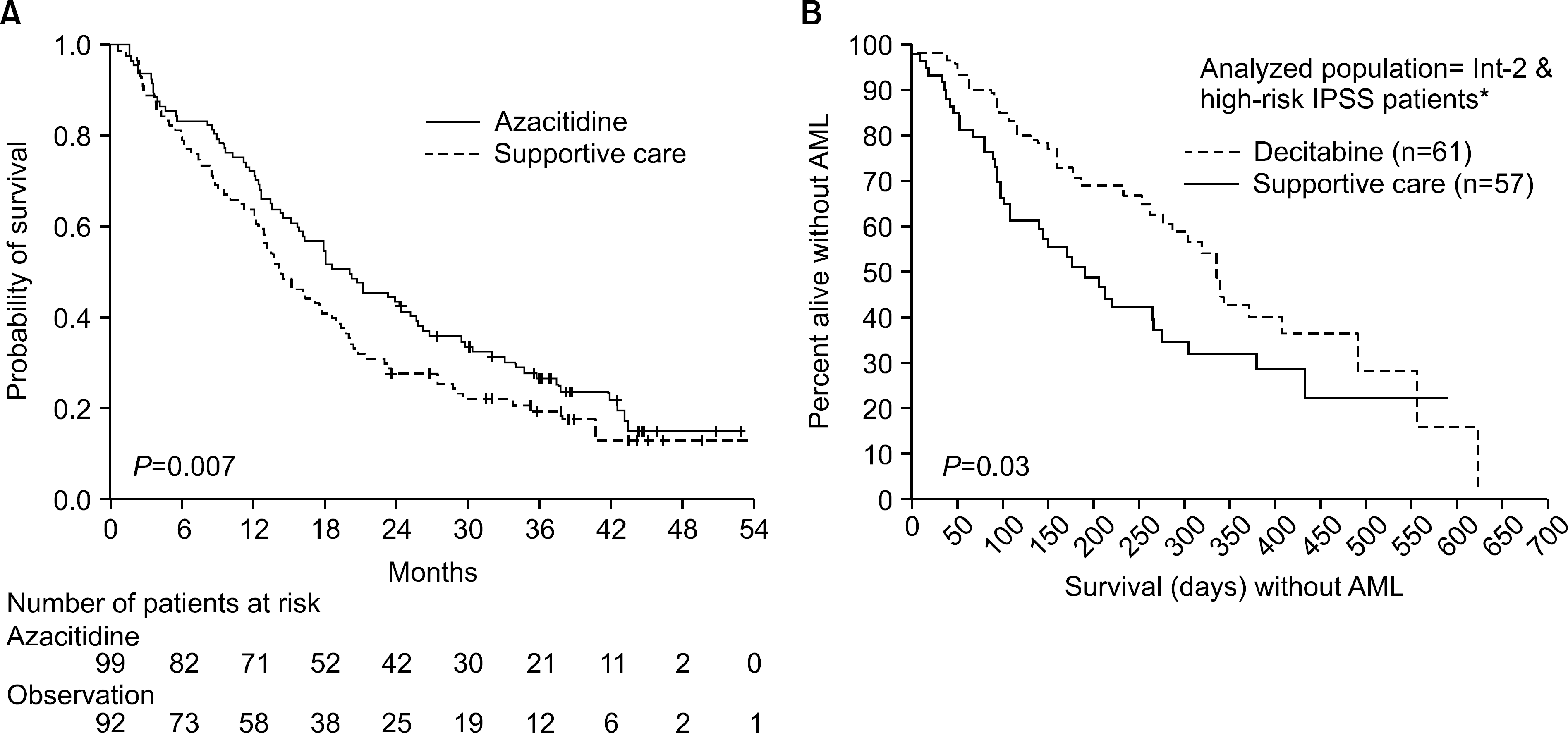
10). Silverman LR., Demakos EP., Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002. 20:2429–40.

11). Kantarjian H., Issa JP., Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodys-plastic syndromes: results of a phase III randomized study. Cancer. 2006. 106:1794–803.
12). Kaminskas E., Farrell A., Abraham S, et al. Approval summary: azacitidine for treatment of myelodys-plastic syndrome subtypes. Clin Cancer Res. 2005. 11:3604–8.

13). Kantarjian H., Oki Y., Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007. 109:52–7.

14). Bowen D., Culligan D., Jowitt S, et al. Guidelines for the diagnosis and therapy of adult myelodysplastic syndromes. Br J Haematol. 2003. 120:187–200.

15). Alessandrino EP., Amadori S., Barosi G, et al. Evidence-and consensus-based practices guidelines for the therapy of primary myelodysplastic syndromes. A statement from the Italian Society of Hematology. Haematologica. 2002. 87:1286–306.
16). Lee JH., Lee JH., Shin YR, et al. Application of different prognostic scoring systems and comparison of the FAB and WHO classifications in Korean patients with myelodysplastic syndrome. Leukemia. 2003. 17:305–13.

17). Schiffer CA. Clinical issues in the management of patients with myelodysplasia. Hematology Am Soc He-matol Educ Program. 2006. 205–10.

18). Cutler CS., Lee SJ., Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004. 104:579–85.

19). Scott BL., Sandmaier BM., Storer B, et al. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia. 2006. 20:128–35.

20). Martino R., Iacobelli S., Brand R, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006. 108:836–46.

21). Castro-Malaspina H., Harris RE., Gajewski J, et al. Unrelated donor marrow transplantation for myelo-dysplastic syndromes: outcome analysis in 510 transplants facilitated by the National Marrow Donor Program. Blood. 2002. 99:1943–51.

22). Deeg HJ. Optimization of transplant regimens for patients with myelodysplastic syndrome (MDS). Hematology Am Soc Hematol Educ Program. 2005. 167–73.

23). Deeg HJ., Storer B., Slattery JT, et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodys-plastic syndrome. Blood. 2002. 100:1201–7.

24). Andersson BS., Kashyap A., Gian V, et al. Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: a phase II study. Biol Blood Marrow Transplant. 2002. 8:145–54.

25). de Lima M., Couriel D., Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004. 104:857–64.

26). Russell JA., Tran HT., Quinlan D, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002. 8:468–76.

27). Ho AY., Pagliuca A., Kenyon M, et al. Reduced-intensity allogeneic hematopoietic stem cell transplantation for myelodysplastic syndrome and acute myeloid leukemia with multilineage dysplasia using fludarabine, busulphan, and alemtuzumab (FBC) conditioning. Blood. 2004. 104:1616–23.

28). Chan GW., Foss FM., Klein AK., Sprague K., Miller KB. Reduced-intensity transplantation for patients with myelodysplastic syndrome achieves durable remission with less graft-versus-host disease. Biol Blood Marrow Transplant. 2003. 9:753–9.

29). de Lima M., Anagnostopoulos A., Munsell M, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004. 104:865–72.

30). Schmid C., Schleuning M., Ledderose G., Tischer J., Kolb HJ. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005. 23:5675–87.

31). de Witte T., Pikkemaat F., Hermans J, et al. Genotypically nonidentical related donors for transplantation of patients with myelodysplastic syndromes: comparison with unrelated donor transplantation and autologous stem cell transplantation. Leukemia. 2001. 15:1878–84.

32). Koh LP., Chao NJ. Umbilical cord blood transplantation in adults using myeloablative and nonmye-loablative preparative regimens. Biol Blood Marrow Transplant. 2004. 10:1–22.

33). Guardiola P., Runde V., Bacigalupo A, et al. Retrospective comparison of bone marrow and granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells for allogeneic stem cell transplantation using HLA identical sibling donors in myelodysplastic syndromes. Blood. 2002. 99:4370–8.

34). Anderson JE. Bone marrow transplantation for myelodysplasia. Blood Rev. 2000. 14:63–77.

35). Fukumoto JS., Greenberg PL. Management of patients with higher risk myelodysplastic syndromes. Crit Rev Oncol Hematol. 2005. 56:179–92.

36). Cheson BD., Greenberg PL., Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006. 108:419–25.

37). Cheson BD., Bennett JM., Kantarjian H, et al. Report of an international working group to standarize response criteria for myelodysplastic syndromes. Blood. 2000. 96:3671–4.
38). List A., Kurtin S., Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005. 352:549–57.

39). Lübbert M., Wijermans P., Kunzmann R, et al. Cytogenetic responses in high-risk myelodysplastic syndrome following low-dose treatment with the DNA methylation inhibitor 5-aza-2'-deoxycytidine. Br J Haematol. 2001. 114:349–57.

40). Lim Z., Killicks S., Cavenagh JD, et al. European multi-centre study on the use of anti-thymocyte globulin in the treatment of myelodysplastic syndromes[ab-stract 2581]. Blood. 2005. 106:707a.
41). Kornblith AB., Hemdon JE., Silverman LR, et al. Impact of azacytidine on the quality of life of patients with myelodysplastic syndrome treated in a randomized phase III trial: a Cancer and Leukemia Group B study. J Clin Oncol. 2002. 20:2441–52.

42). Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999. 341:1986–95.

43). Piperno A. Classification and diagnosis of iron overload. Haematologica. 1998. 83:447–55.
44). Gabuti V., Piga A. Results of long-term iron-chelating therapy. Acta Haematol. 1996. 95:26–36.

45). Olivieri NF., Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997. 89:739–61.

46). Ishizaka N., Saito K., Mitani H, et al. Iron overload augments angiotensin II-induced cardiac fibrosis and promotes neointima formation. Circulation. 2002. 106:1840–6.

47). Jensen PD. Evaluation of iron overload. Br J Hae-matol. 2004. 124:697–711.

48). Porter JB. Practical management of iron overload. Br J Haematol. 2001. 115:239–52.

49). Angelucci E., Brittenham GM., McLaren CE, et al. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med. 2000. 343:327–31.

50). Liu ZD., Hider RC. Design of clinically useful iron (III)-selective chelators. Med Res Rev. 2002. 22:26–64.
51). Piga A., Galanello R., Forni GL, et al. Randomized phase II trial of deferasirox (Exjade, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overload. Haematologica. 2006. 91:873–80.
52). Greenberg PL. Myelodysplastic syndromes: iron overload consequences and current chelating therapies. J Natl Compr Canc Netw. 2006. 4:91–6.

53). Hasle H., Niemeyer CM., Chessells JM, et al. A pediatric approach to the WHO classification of myelodys-plastic and myeloproliferative diseases. Leukemia. 2003. 17:277–82.

54). Hasle H. Myelodysplastic and myeloproliferative disorders in children. Curr Opin Pediatr. 2007. 19:1–8.

55). Mandel K., Dror Y., Poon A., Freedman MH. A practical, comprehensive classification for pediatric mye-lodysplastic syndromes: the CCC system. J Pediatr Hematol Oncol. 2002. 24:343–52.

56). Occhipinti E., Correa H., Yu L., Craver R. Comparison of two new classifications for pediatric myelodys-plastic and myeloproliferative disorders. Pediatr Blood Cancer. 2005. 44:240–4.

57). Niemeyer CM., Locatelli F. Chronic myeloproliferative disorders. Pui CH, editor. Childhood leukemias. 2nd ed.Cambridge, England: Cambridge University Press;2006. p. 571–98.

58). Kook H., Kim MK., Ghim TT, et al. Pediatric myelo-dysplastic syndrome in Korea: Clinical characteristics and comparison of prognostic scoring systems. Korean J Pediatr Hematol-Oncol. 2003. 10:1–13.
59). Woods WG., Barnard DR., Alonzo TA, et al. Prospective study of 90 children requiring treatment for juvenile myelomonocytic leukemia or myelodys-plastic syndrome: a report from the Children's Cancer Group. J Clin Oncol. 2002. 20:434–40.

60). Webb DK., Passmore SJ., Hann IM., Harrison G., Wheatley K., Chessells JM. Results of treatment of children with refractory anaemia with excess blasts (RAEB) and RAEB in transformation (RAEBt) in Great Britain 1990-99. Br J Haematol. 2002. 117:33–9.

61). Yusuf U., Frangoul HA., Gooley TA, et al. Allogeneic bone marrow transplantation in children with myelo-dysplastic syndrome or juvenile myelomonocytic leukemia: the Seattle experience. Bone Marrow Transplant. 2004. 33:805–14.

62). Smith FO., Woods WG. Myeloproliferative and mye-lodysplastic disorders. Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. 5th ed.Philadelphia, USA: Lippincott Williams & Wilkins;2006. p. 673–94.
63). Kang HJ., Shin HY., Choi HS., Ahn HS. Novel regimen for the treatment of juvenile myelomonocytic leukemia (JMML). Leuk Res. 2004. 28:167–70.

64). Smith FO., King R., Nelson G, et al. Unrelated donor bone marrow transplantation for children with juvenile myelomonocytic leukaemia. Br J Haematol. 2002. 116:716–24.

65). Locatelli F., Nöllke P., Zecca M, et al. Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood. 2005. 105:410–9.

66). Vidal DO., Paixão VA., Brait M, et al. Aberrant methylation in pediatric myelodysplastic syndrome. Leuk Res. 2007. 31:175–81.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download