Abstract
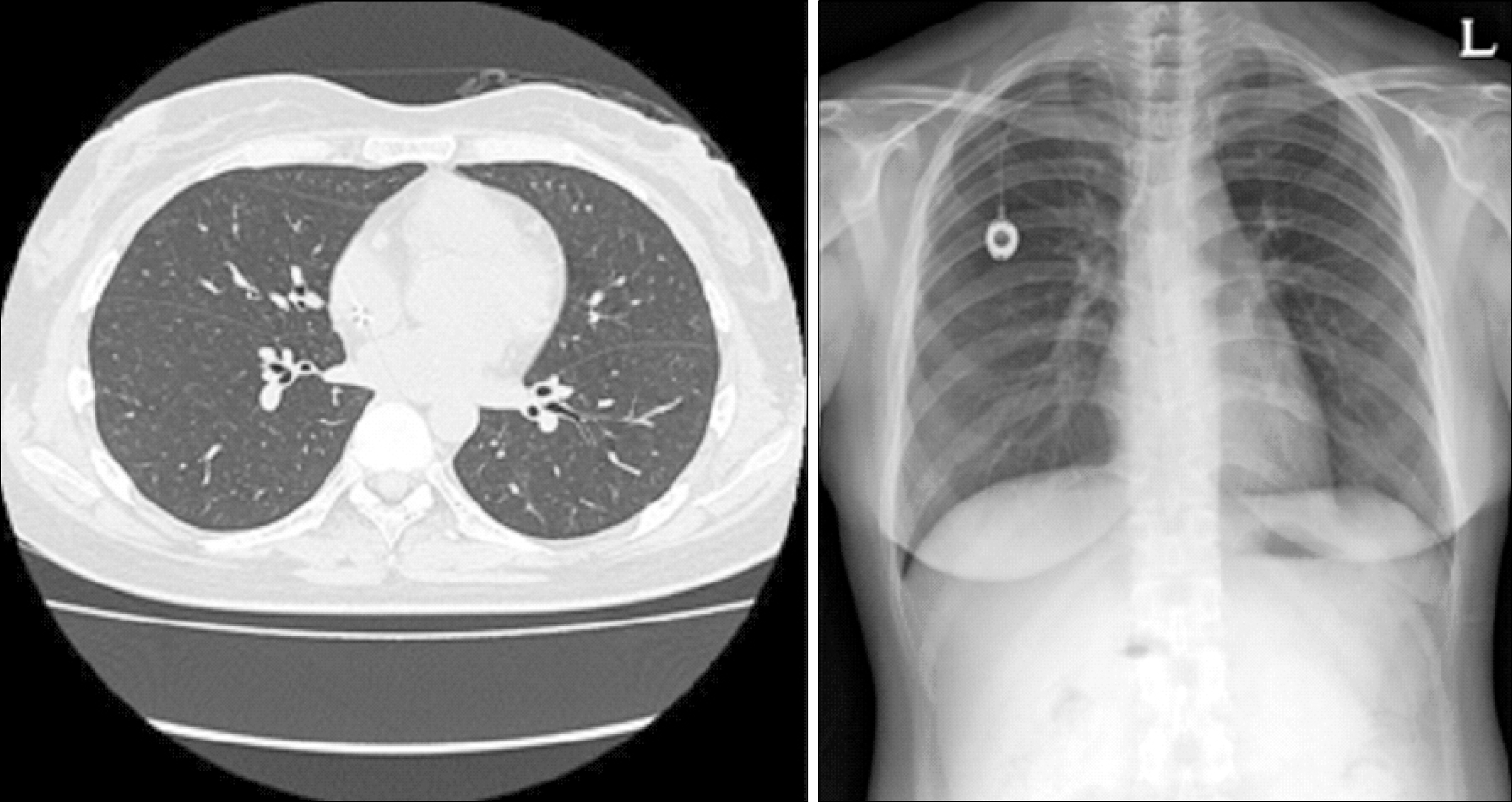
Side effects of rituximab are mild in most cases, but there have been a few cases of severe pulmonary toxicity reported in elderly patients. Here we report a case of interstitial pneumonitis following rituximab treatment in a young patient. A 35-year-old woman with diffuse large B-cell lymphoma was admitted complaining of dry cough and dyspnea without fever after the 3 treatments with rituximab-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) chemotherapy. Her chest CT with high-resolution CT scanning confirmed the presence of bilateral diffuse ground-glass opacities. The analysis of arterial blood gases indicated hypoxemia. The pulmonary function testing showed a restrictive pattern. There were no other findings suggesting an infection. The findings were compatible with a rituximab-induced interstitial pneumonitis. After the patient was treated with prednisolone, the symptoms resolved. Cases with rituximab-induced interstitial pneumonitis develop principally in elderly patients. However, the condition also can occur in young patients.
Go to : 
REFERENCES
1). Maloney DG., Smith B., Rose A. Rithuximab: mechanism of action and resistance. Semin Oncol. 2002. 29(2 Suppl):2–9.
3). Dass S., Vital EM., Emery P. Rituximab: novel B-cell depletion therapy for the treatment of rheumatoid arthritis. Expert Opin Pharmacother. 2006. 7:2559–70.

4). Coiffier B., Lepage E., Brière J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002. 346:235–42.

5). Enomoto T., Usuki J., Azuma A., Nakagawa T., Kudoh S. Diabetes mellitus may increase risk for idiopathic pulmonary fibrosis. Chest. 2003. 123:2007–11.
6). Limper AH. Drug-induced pulmonary disease. In: Mason RJ, Murray JF, Broaddus VC, Nadel JA, eds. 4th ed.Text book of respiratory medicine. Philadelphia: Elsevier Saunders;2005. p. 611–24.
7). Burton C., Kaczmarski R., Jan-Mohamed R. Interstitial pneumonitis related to rituximab therapy. N Engl J Med. 2003. 348:2690–1.

8). Herishanu Y., Polliack A., Leider-Trejo L., Grieff Y., Metser U., Naparstek E. Fatal interstitial pneumonitis related to rituximab-containing regimen. Clin Lymphoma Myeloma. 2006. 6:407–9.

9). Bienvenu J., Chvetzoff R., Salles G, et al. Tumor necrosis factor alpha release is a major biological event associated with rituximab treatment. Hematol J. 2001. 2:378–84.
10). Kanelli S., Ansell SM., Habermann TM., Inwards DJ., Tuinstra N., Witzig TE. Rituximab toxicity in patients with peripheral blood mailgnant B-cell lymphocytosis. Leuk Lymphoma. 2001. 42:1329–37.
11). Alexandrescu DT., Dutcher JP., O'Boyle K., Albulak M., Oiseth S., Wiernik PH. Fatal intra-alveloar hemorrhage after rituximab in a patient with non-Hodg-kin's lymphoma. Leuk Lymphoma. 2004. 45:2321–5.
12). Hiraga J., Kondoh Y., Taniguchi H., Kinoshita T., Naoe T. A case of interstitial pneumonia induced by rithuximab therapy. Int J Hematol. 2005. 81:169–70.
13). Choi YJ., Jung WJ., Oh SI, et al. A case of interstitial lung diseasec caused by Rituximab in non-Hodgkin lymphoma. Korean J Med. 2006. 71:449–55.
Go to : 
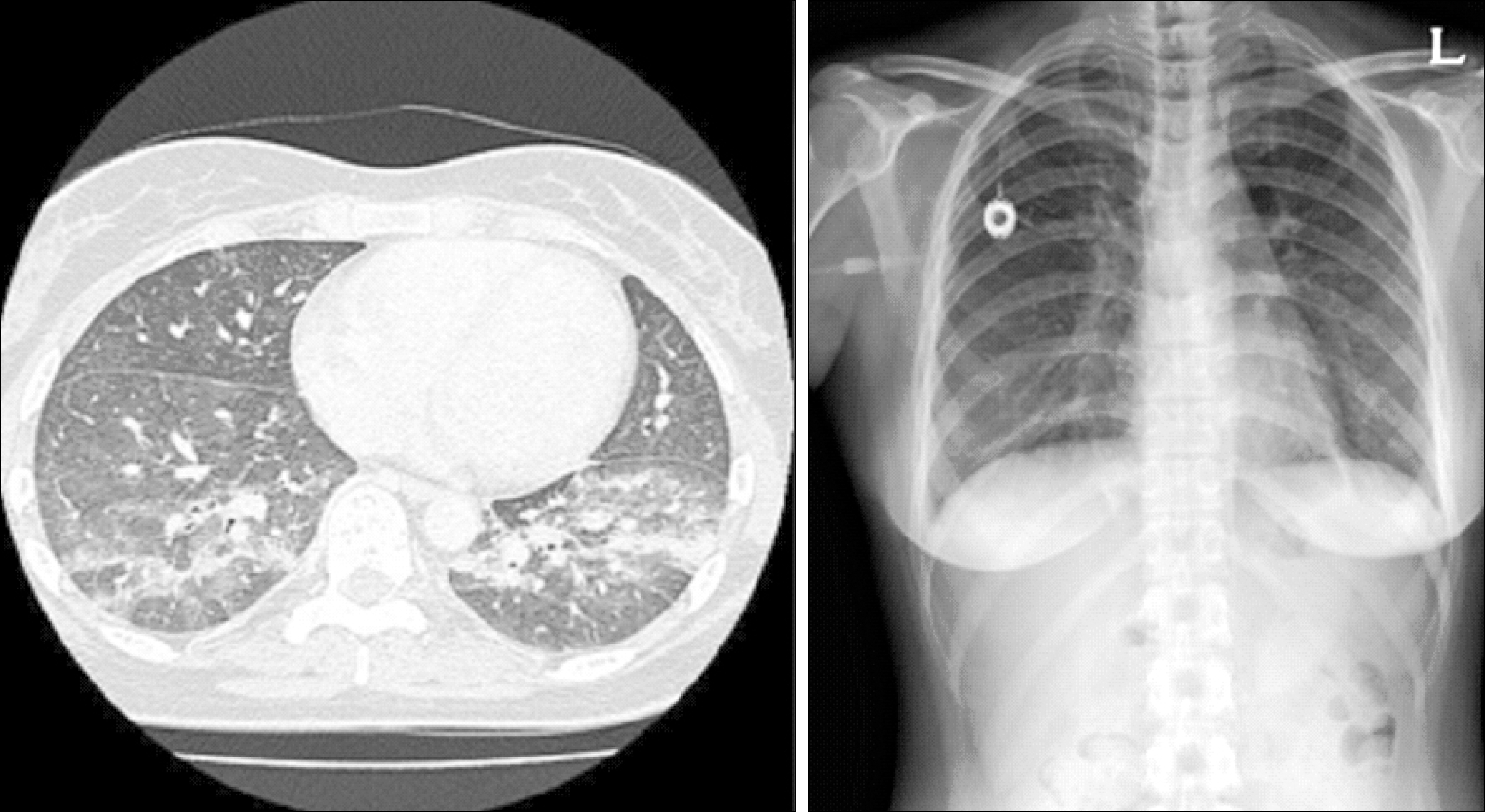
 | Fig. 2After prednisolone treatment for 2 weeks, interstitial pneumonitis resolved in chest HRCT scan and radiography. |
Table 1.
Analysis of arterial blood gases before and afte prednisolone treatment
| Parameter | Before treatment | After treatment |
|---|---|---|
| pH | 7.449 | 7.427 |
| pCO2 (mmHg) | 38.0 | 33.0 |
| pO2 (mmHg) | 66.4 | 130.6 |
| O2 saturation (%) | 94.0 | 98.7 |
Table 2.
Pulmonary function tests before and after prednisolone treatment
Table 3.
Patients’ characteristics of reported cases




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download