Abstract
Background:
Blood typing is an essential test for transfusion. Generally, blood typing is performed using a slide test, tube test or microcolumn agglutination test. The aims of this study were to develop a new blood typing kit using micromachining, microfluidics and microseparation methods, and to evaluate the clinical usefulness of the new blood typing kit.
Methods:
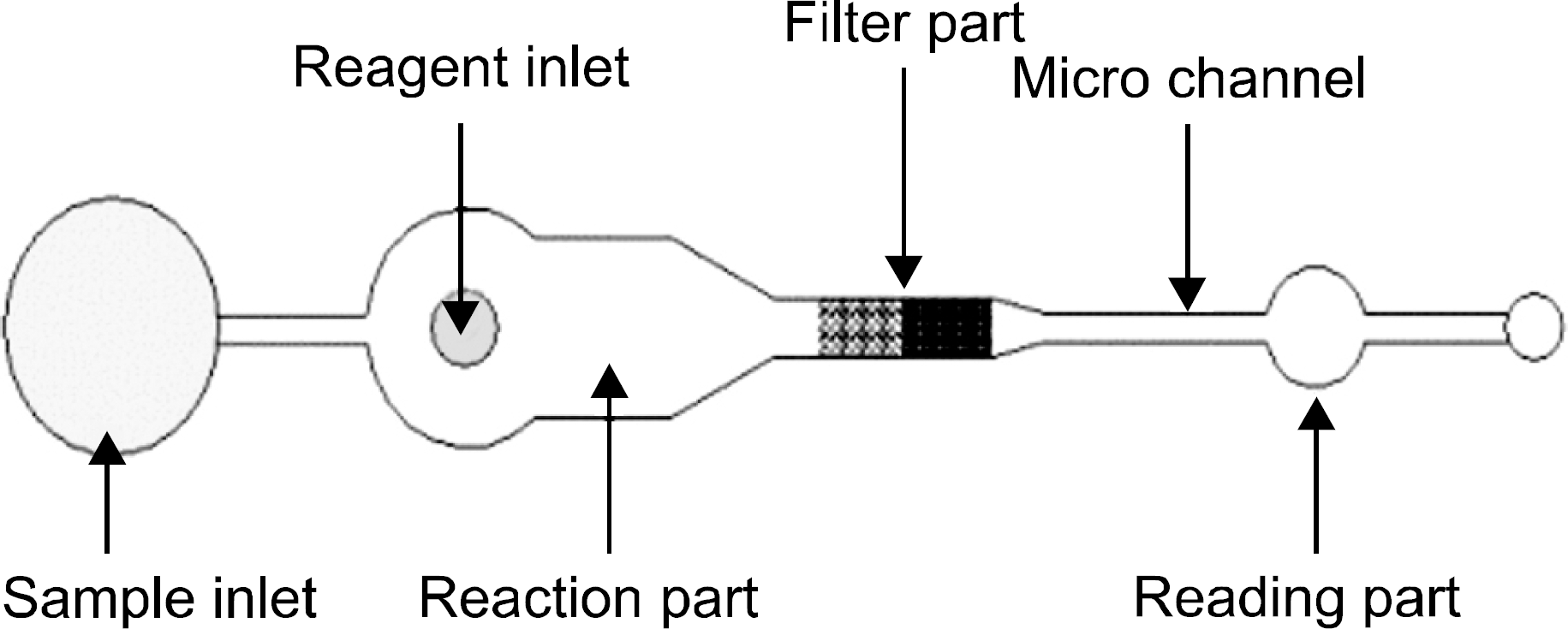
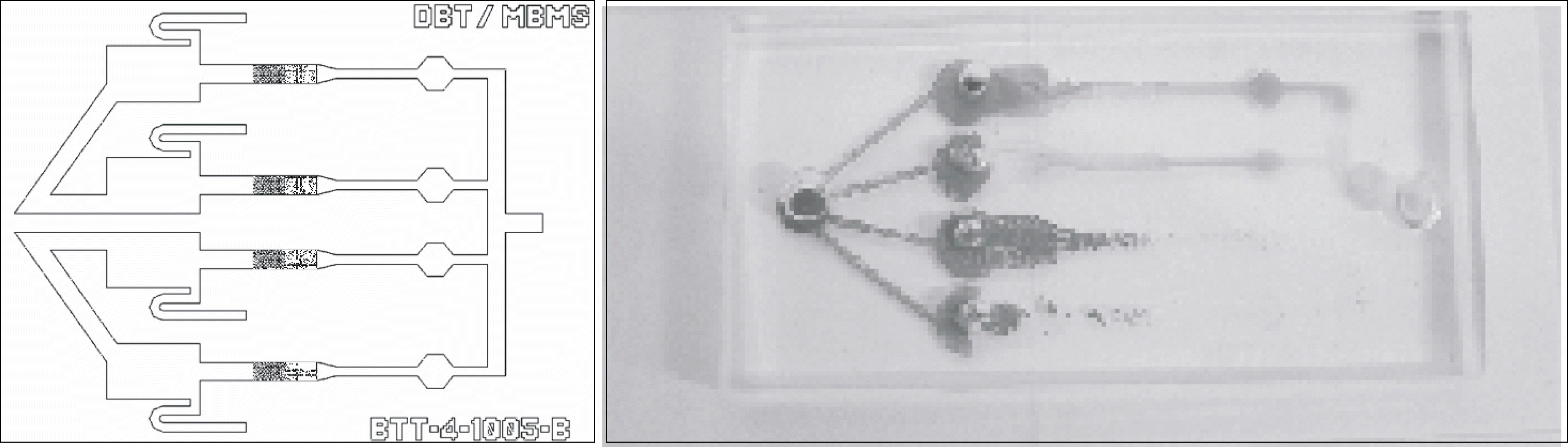
We designed and manufactured a blood typing microchip using polydimethylsiloxane (PDMS), which contained a microchannel (25∼200μm). The blood sample and antisera to be tested were dropped on the microwell for movement and mixing by capillary action. Once agglutination occurred, the microchannel acts as a filter and the blood type was determined by observation by the naked eye. To evaluate the newtyping kit, we tested sensitivity using artificially diluted blood and compared the results of the new typing method with the slide and tube methods using 70 samples.
Results:
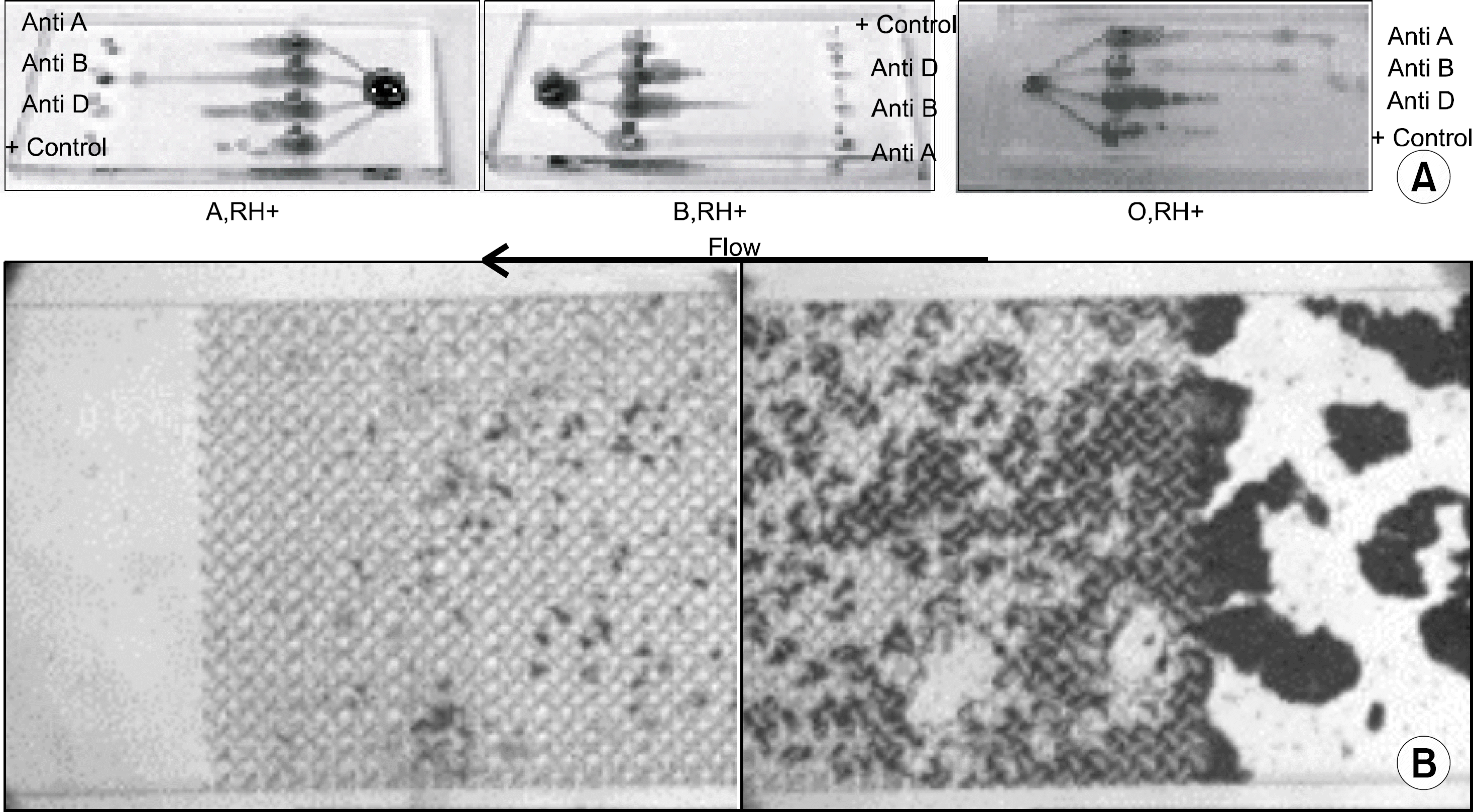
The new blood typing kit could differentiate a +4∼+2 agglutination reaction, but could not detect a +1 agglutination reaction as observed by the naked eye. Among 70 samples, the results of ABO and Rh typing by the new typing method (n=66, ≥+2 agglutination reaction by the column agglutination method) were in accord with the results of the tube and slide methods, but couldnot detect agglutination in all 4 clinical samples, below a +1 agglutination reaction.
Conclusion:
The new blood typing kit is inadequate for routine use in the clinical laboratory due to low sensitivity, but with further improvement, it can be used economically, conveniently and objectively for blood typing without any special equipment. Moreover, the microfludics and separation method may be broadly applicable in other tests using the hemagglutination method.
Go to : 
REFERENCES
1). Landsteiner K. Agglutination phenomena of normal human blood. Wien Klin Wochenschr. 2001. 113:768–9.
2). Shin JW., Jeong SH., Nahm CH., Kim HY., Kwon OH. The direct antiglobulin test and antibody screening test based on the antiglobulin gel technique. Korean J Clin Pathol. 1996. 16:411–8.
3). Chang SH., Lee NY., Choi YC., Yoo BJ., Suh JS. Irregular antibody screening in cord blood by column agglutination test. Korean J Blood Transfus. 1997. 8:65–72.
4). Nilsson A., Petersson F., Jonsson H., Laurell T. Acoustic control of suspended particles in micro fluidic chips. Lab Chip. 2004. 4:131–5.

5). Petersson F., Nilsson A., Holm C., Jonsson H., Laurell T. Separation of lipids from blood utilizing ultrasonic standing waves in microfluidic channels. Analyst. 2004. 129:938–43.

6). Whitworth G., Grundy MA., Coakley WT. Transport and harvesting of suspended particles using modulated ultrasound. Ultrasonics. 1991. 29:439–44.

7). Ziaie B., Baldi A., Lei M., Gu Y., Siegel RA. Hard and soft micromachining for BioMEMS: review of techniques and examples of applications in microfluidics and drug delivery. Adv Drug Deliv Rev. 2004. 56:145–72.

9). Kim YD., Park CB., Clark DS. Stable sol-gel microstructured and microfluidic networks for protein patterning. Biotechnol Bioeng. 2001. 73:331–7.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download