Abstract
Background:
Growth impairment is a common complication after hematopoietic stem cell transplantation (SCT). The aim of this study was to evaluate the final adult height of patients who underwent SCT in childhood and to identify the factors that influence long-term growth in these patients.
Methods:
A retrospective review of 15 children who underwent SCT before puberty at Chonnam National University Hospital and reached final adult height was undertaken. To assess the severity of height reduction and to monitor the height changes longitudinally, height measurements of each patient both at the time of SCT and the final height were expressed as the height standard deviation score (SDS).
Results:
Seven children were males and eight were females with a median age of 12.8±2.4 years (range, 6.3∼14.7) at SCT. The median follow-up period was 7.1±2.0 years (range, 4.5∼11.1) and their final height was achieved at 18.1±1.5 years (range, 17.0∼21.8). Final height SDS values were within normal for the healthy population in all except two who had short stature (below ?2.0 SDS). No patient achieved height values greater than +2.0 SDS. The final height SDS value (?0.5±1.2) was not decreased from the height SDS value at SCT (?0.8±0.8). The younger age group at SCT (6.1∼10.0 years, n=5) showed significantly lower final height SDS and greater ΔSDS than the older age group (10.1∼15.0 years, n=10) (?1.5±0.6 vs. ?0.1±1.1, P<.05; ?1.2±0.7 vs. 0.5±0.8, P<.05, respectively). The irradiation-based conditioning (n=6) had negative effects on the ΔSDS (P>.05) and the final height SDS (P<.05). The gender, type of disease, donor type or the presence of chronic graft-versus-host disease did not influence height.
REFERENCES
1). Cohen A., Rovelli A., Bakker B, et al. Final height of patients who underwent bone marrow transplantation for hematological disorders during childhood: a study by the Working Party for Late Effects-EBMT. Blood. 1999. 93:4109–15.
2). Korean Society of Pediatric Endocrinology. Growth. Pediatric endocrinology. 2nd ed.Korea: Kwangmun Press;2004. p. 36–56.
3). Cohen A., Rovelli A., Van-Lint MT, et al. Final height of patients who underwent bone marrow transplantation during childhood. Arch Dis Child. 1996. 74:437–40.

4). Clement-De Boers A., Oostdijk W., Van Weel-Sipman MH., Van den Broeck J., Wit JM., Vossen JM. Final height and hormonal function after bone marrow transplantation in children. J Pediatr. 1996. 129:544–50.
5). Huma Z., Boulad F., Black P., Heller G., Sklar C. Growth in children after bone marrow transplantation for acute leukemia. Blood. 1995. 86:819–24.

6). Bakker B., Massa GG., Oostdijk W., Weel-Sipman MH., Vossen JM., Wit JM. Pubertal development and growth after total-body irradiation and bone marrow transplantation for haematological malignancies. Eur J Pediatr. 2000. 159:31–7.

7). Bakker B., Oostdijk W., Geskus RB., Stokvis-Brantsma WH., Vossen JM., Wit JM. Patterns of growth and body proportions after total-body irradiation and hematopoietic stem cell transplantation during childhood. Pediatr Res. 2006. 59:259–64.

8). Sanders JE., Guthrie KA., Hoffmeister PA., Woolfrey AE., Carpenter PA., Appelbaum FR. Final adult height of patients who received hematopoietic cell transplantation in childhood. Blood. 2005. 105:1348–54.

9). Cohen A., van Lint MT., Uderzo C, et al. Growth in patients after allogeneic bone marrow transplantation for hematological diseases in childhood. Bone Marrow Transplant. 1995. 15:343–8.
10). Tanner JM., Davis PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985. 107:317–29.

11). Choi JM. 2005 Standards for growth for Korean child and adolescence. The 56th Korean Pediatric Annual Meeting Abstract Book; 2005 Apr 28-29; Yongpyung. Seoul: Korean Pediatric Society. 2005.
12). Couto-Silva AC., Trivin C., Esperou H., Michon J., Fischer A., Brauner R. Changes in height, weight, and plasma leptin after bone marrow transplantation. Bone Marrow Transplant. 2000. 26:1205–10.

13). Holm K., Nysom K., Rasmussen MH, et al. Growth, growth hormone and final height after BMT. Possible recovery of irradiation-induced growth hormone insufficiency. Bone Marrow Transplant. 1996. 18:163–70.
14). Shalet SM., Brennan BM. Growth and growth hormone status after a bone marrow transplantation. Horm Res. 2002. 58(Suppl 1):86–90.
15). Shinagawa T., Tomita Y., Ishiguro H, et al. Final height and growth hormone secretion after bone marrow transplantation in children. Endocr J. 2001. 48:133–8.

16). Shalet SM., Brennan BM., Reddingius RE. Growth hormone therapy and malignancy. Horm Res. 1997. 48(Suppl 4):29–32.

17). Jenkins PJ., Mukherjee A., Shalet SM. Does growth hormone cause cancer? Clin Endocrinol. 2006. 64:115–21.

18). Leung W., Rose SR., Zhou Y, et al. Outcomes of growth hormone replacement therapy in survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2002. 20:2959–64.

19). Katz JA., Pollock BH., Jacaruso D., Morad A. Final height attained in patients successfully treated for acute lymphoblastic leukemia. J Pediatr. 1993. 123:546–52.
Fig. 2
Final SDS (SDS at final height) and ΔSDS (final height SDS-SDS at transplantation) in different age groups. Younger age group (<10 years) shows lower final and ΔSDS than older group (>10 years).
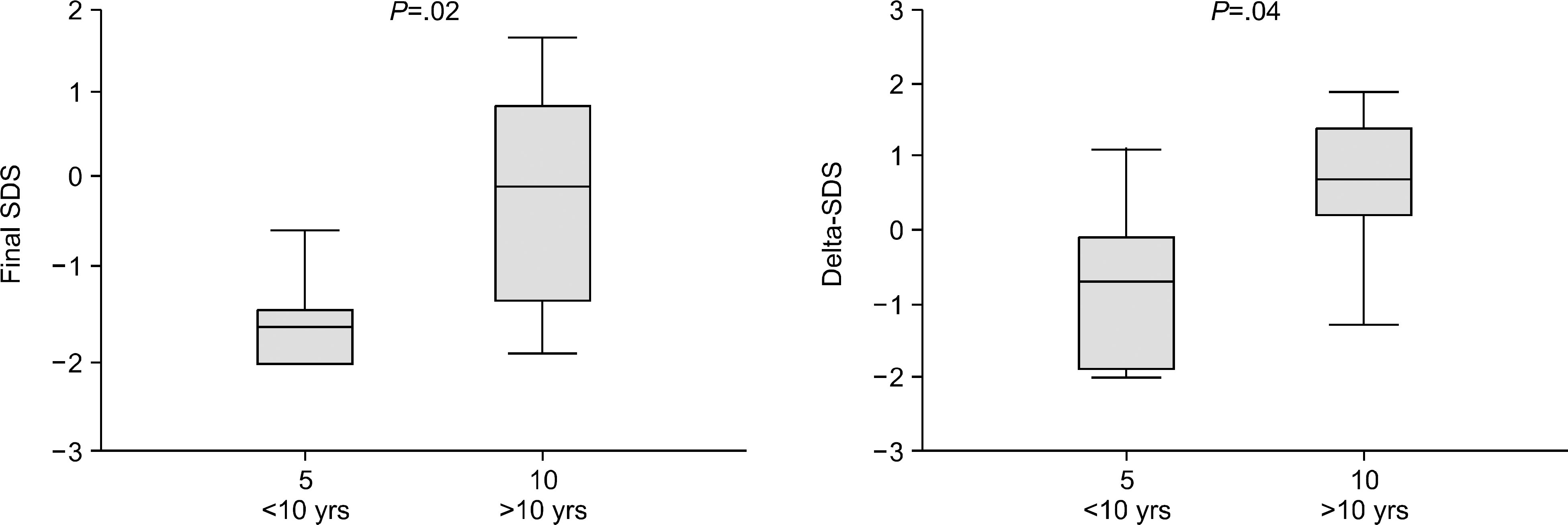
Fig. 3
ΔSDS (final height SDS-SDS at transplantation) in different irradiation groups. (A) Irradiation group shows lower ΔSDS than non-irradiation group. (B) ΔSDS is the lowest in patients who received TBI, followed by TLI group and no irradiation group.
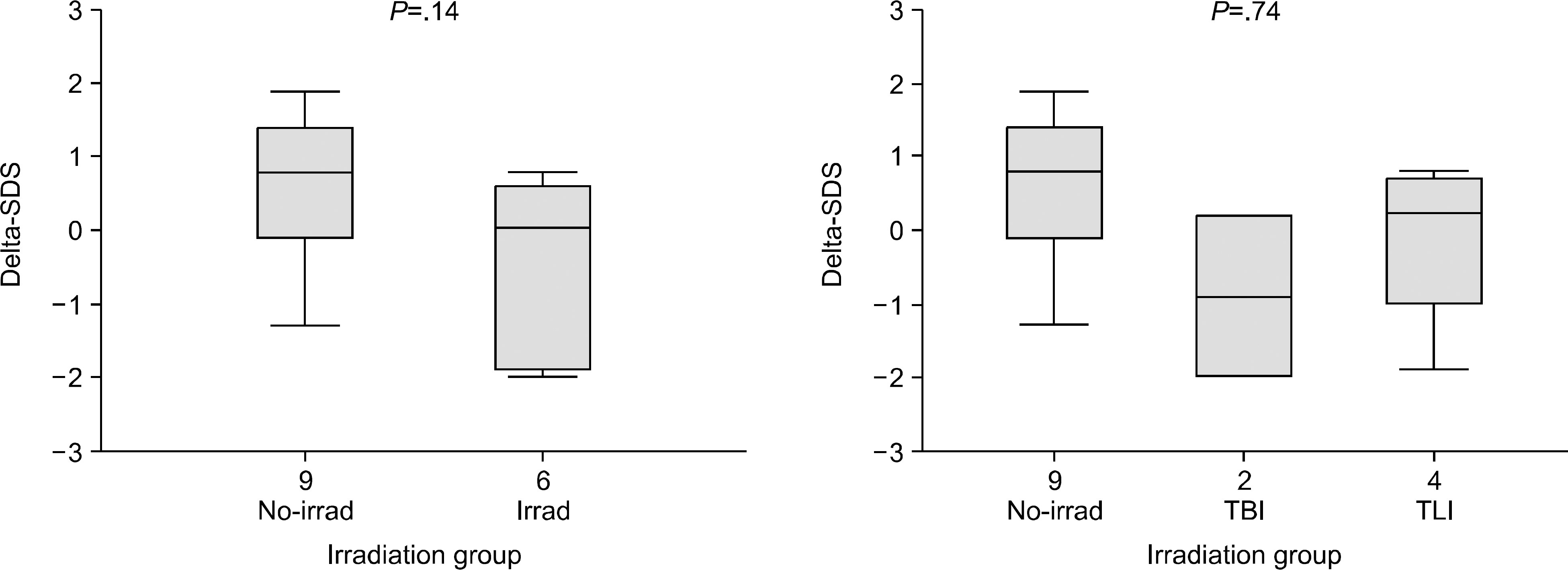
Table 1.
Clinical profiles of children at stem cell transplantation
Table 2.
Stem cell transplantation and height SDS
∗Patients with short stature. Abbreviations: AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; Allo, allogeneic; Ara-C, cytosine arabinoside; Auto, autologous; Bu, busulfan; Cy, cyclophosphamide; mVAMP, modified teniposide, adriamycin, melphalan, carboplatin; NB, neuroblastoma; SAA, severe aplastic anemia; SCT, stem cell transplantation; TBI, total body irradiation; TLI, total lymphoid irradiation; VP-16, etoposide.
Table 3.
Final SDS and ΔSDS by clinical parameters




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download