Abstract
Background:
Most of adult patients with chronic immune thrombocytopenic purpura (ITP) that was refractory or relapsed to high-dose corticosteroid have been treated with splenectomy as a 2nd line treatment. However, these patients may have increased morbidity and mortality according to the operation and the increased risk of infection for a lifetime after splenectomy. Despite of the above risks, 30∼40% of these patients can’t maintain remission. Furthermore, the remission rate after splenectomy is relatively lower in patients with corticosteroid-refractory chronic ITP than that in those patients with corticosteroid-responsiveness. We studied whether danazol, an attenuated androgen, is useful or safe as 2nd line treatment for chronic ITP instead of splenectomy and which factors are associated with the response to danazol.
Methods:
Among the patients with chronic ITP who failed corticosteroid therapy in our hospital, 28 patients who received danazol as the 2nd line treatment were analyzed retrospectively. A complete response was defined that the platelet count was increased to 150×103/μL, and a partial response was defined that the platelet count was increased above 50×103/μL or there was an increased platelet count of more than 20×103/μL from the pre-treatment platelet count when the platelet count was above 50× 103/μL at the time of danazol therapy.
Results:
The median age of patients was 44 years (range: 19∼67) and the number of male patients was 9 (32.1%) and the number of females was 19 (67.9%). The starting daily doses of danazol were variable from 200 to 600mg, though most of the patients were treated with 400mg daily (18 cases, 64.3%). The median duration of danazol therapy was 201.5 days (range: 13∼973) and the median duration from ITP diagnosis to danazol treatment was 56 days (range: 20∼2,430). Among the accrued 28 patients, 22 patients showed a response to danazol (78.5%); there were 6 patients (21.4%) with a complete response and 16 patients (57.1%) with a partial response. The median duration from danazol treatment to response was 30 days (range: 0∼180). The median response duration of danazol treatment was 330 days (95%
CI:
182∼478) by the Kaplan-Meiyer method. For the danazol-responsive patients, 9 patients (40.9%) remained in remission and 13 patients (59.1%) relapsed. Grade 3∼4 toxicity was observed in two patients and three patients stopped danazol because of adverse effects. Hepatotoxicity was the most common toxicity.
REFERENCES
2). James B., Douglas C. Immune thrombocytopenic purpura, neonatal alloimmune thrombocytopenia, and post-thransfusion purpura. Ronald H, editor. Hematology-basic principles and practice. 4th ed.Philadelphia: Elsevier Inc;2005. p. 2269–85.
3). Gernsheimer T., Stratton J., Ballem PJ., Slichter SJ. Mechanisms of response to treatment in autoimmune thrombocytopenic purpura. N Engl J Med. 1989. 320:974–80.

4). Doan CA., Bouroncle BA., Wiseman BK. Idiopathic and secondary thrombocytopenic purpura: clinical study and evaluation of 381 cases over a period of 28 years. Ann Intern Med. 1960. 53:861–76.

5). Choi CW., Yoon SY., Paek CW, et al. Effect of Splenectomy in Adult Patients with Idiopathic Thrombocytopenic Purpura (ITP). Korean J Hematol. 1999. 34:513–20.
6). Kojouri K., Vesely SK., Terrell DR., George JN. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood. 2004. 104:2623–34.

7). Maloisel F., Andres E., Zimmer J, et al. Danazol therapy in patients with chronic idiopathic thrombocytopenic purpura: long-term results. Am J Med. 2004. 116:590–4.

8). Picozzi VJ., Roeske WR., Creger WP. Fate of therapy failures in adult idiopathic thrombocytopenic purpura. Am J Med. 1980. 69:690–4.

9). Fabris F., Tassan T., Ramon R, et al. Age as the major predictive factor of long-term response to splenectomy in immune thrombocytopenic purpura. Br J Haematol. 2001. 112:637–40.

10). Andres E., Zimmer J., Noel E., Kaltenbach G., Koumarianou A., Maloisel F. Idiopathic thrombocytopenic purpura: a retrospective analysis in 139 patients of the influence of age on the response to corticosteroids, splenectomy and danazol. Drugs Aging. 2003. 20:841–6.
11). Ahn YS., Rocha R., Mylvaganam R., Garcia R., Duncan R., Harrington WJ. Long-term danazol therapy in autoimmune thrombocytopenia: unmaintained remission and age-dependent response in women. Ann Intern Med. 1989. 111:723–9.

12). Ahn YS., Harrington WJ., Simon SR., Mylvaganam R., Pall LM., So AG. Danazol for the treatment of idiopathic thrombocytopenic purpura. N Engl J Med. 1983. 308:1396–9.

13). Portielje JE., Westendorp RG., Kluin-Nelemans HC., Brand A. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood. 2001. 97:2549–54.

14). Cortelazzo S., Finazzi G., Buelli M., Molteni A., Viero P., Barbui T. High risk of severe bleeding in aged patients with chronic idiopathic thrombocytopenic purpura. Blood. 1991. 77:31–3.

15). Schreiber AD., Chien P., Tomaski A., Cines DB. Effect of Danazol in immune thrombocytopenic purpura. N Engl J Med. 1987. 316:503–8.

16). Cines DB., McKenzie SE., Siegel DL. Mechanisms of action of therapeutics in idiopathic thrombocytopenic purpura. J Pediatr Hematol Oncol. 2003. 25(Suppl 1):S52–6.

17). Fenaux P., Quiquandon I., Huart JJ., Caulier MT., Bauters F. The role of danazol in the treatment of refractory idiopathic thrombocytopenic purpura. A report of 22 cases. Nouv Rev Fr Hematol. 1990. 32:143–6.
18). Ruberto E., Espinola R. Treatment of idiopathic thrombocytopenic purpura with danazol. Sangre. 1995. 40:307–10.
19). Schiavotto C., Castaman G., Rodeghiero F. Treatment of idiopathic thrombocytopenic purpura(ITP) in patients with refractoriness to or with contraindication for corticosteroids and/or splenectomy with immunosuppressive therapy and danazol. Haema-tologica. 1993. 78:29–34.
20). Hasegawa Y., Nagasawa T., Kojima H., Shibuya A., Ninomiya H., Abe T. Treatment of elderly patients with chronic idiopathic thrombocytopenic purpura. Rinsho Ketsueki. 1993. 34:460–4.
21). Pakhale S., Moltyaner Y., Chamberlain D., Lazar N. Rapidly progressive pulmonary fibrosis in a patient treated with danazol for idiopathic thrombocytopenic purpura. Can Repir J. 2004. 11:55–7.

22). Shah A., Roberts T., McQueen IN., Graham JG., Walker K. Danazol and benign intracranial hypertension. Br Med J (Clin Res Ed). 1987. 294:1323.

23). Confavreux C. Seve P, Broussolle C, Renaudier P, Ducerf C. Danazol-induced hepatocellular carcinoma. QJM. 2003. 96:317–8.
24). Kondo H., Iseki T., Goto S., Takaso T., Ohto M., Okuda K. Danazol therapy in idiopathic thrombocytopenic purpura: the efficacy of low-medium dose therapy. Int J Hematol. 1992. 55:293–300.
Fig. 1
Event-free survival in 28 patients with danazol therapy. An event was defined by the lack of response, relapse, and major side effect leading to drug withdrawal. Survival was calculated from the first day of danazol therapy to the day of the event.
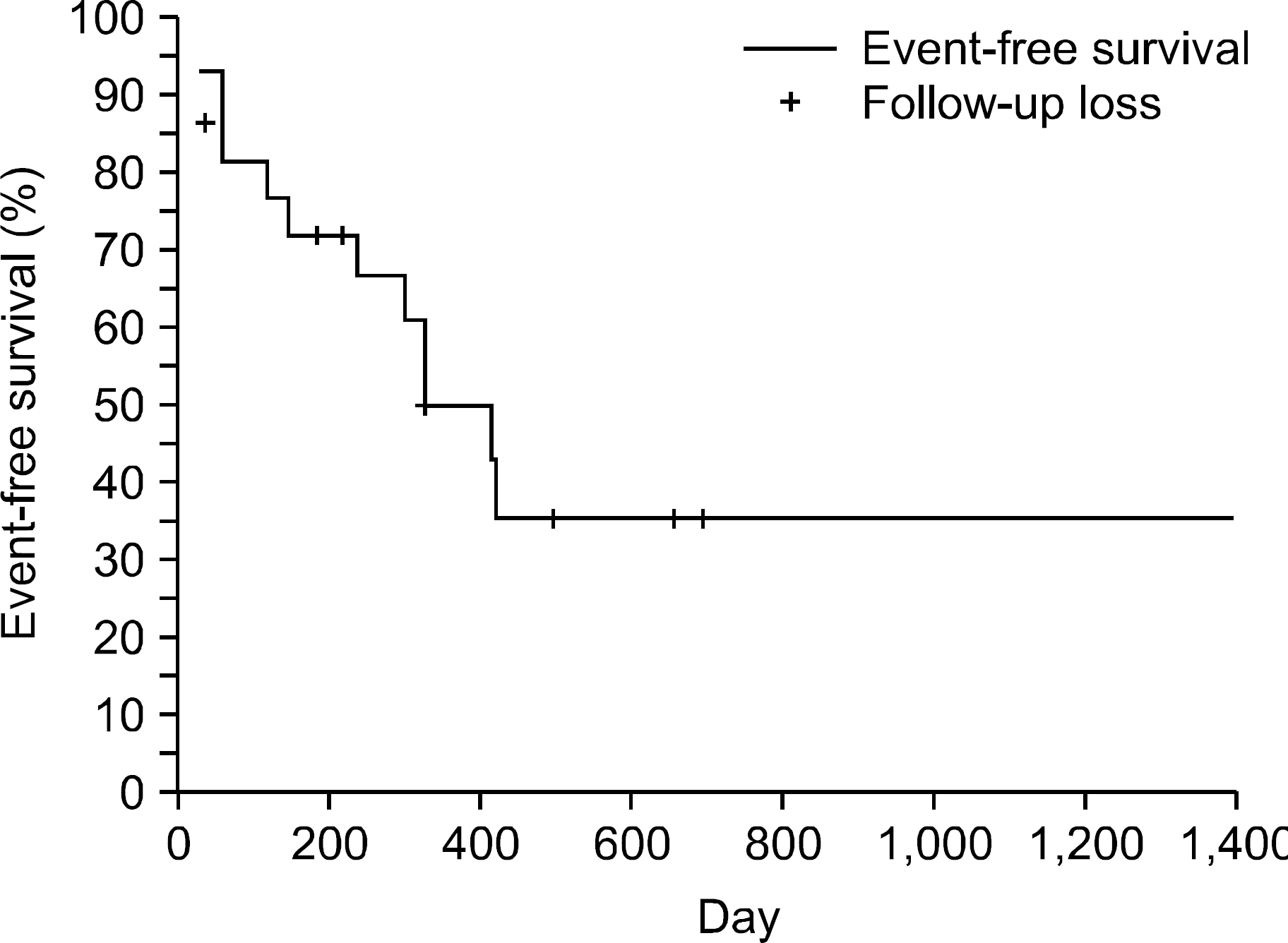
Fig. 2
Time to response in 22 patients with the response to danazol. This was defined from the first day of danazol therapy to the day of the first partial or complete response.
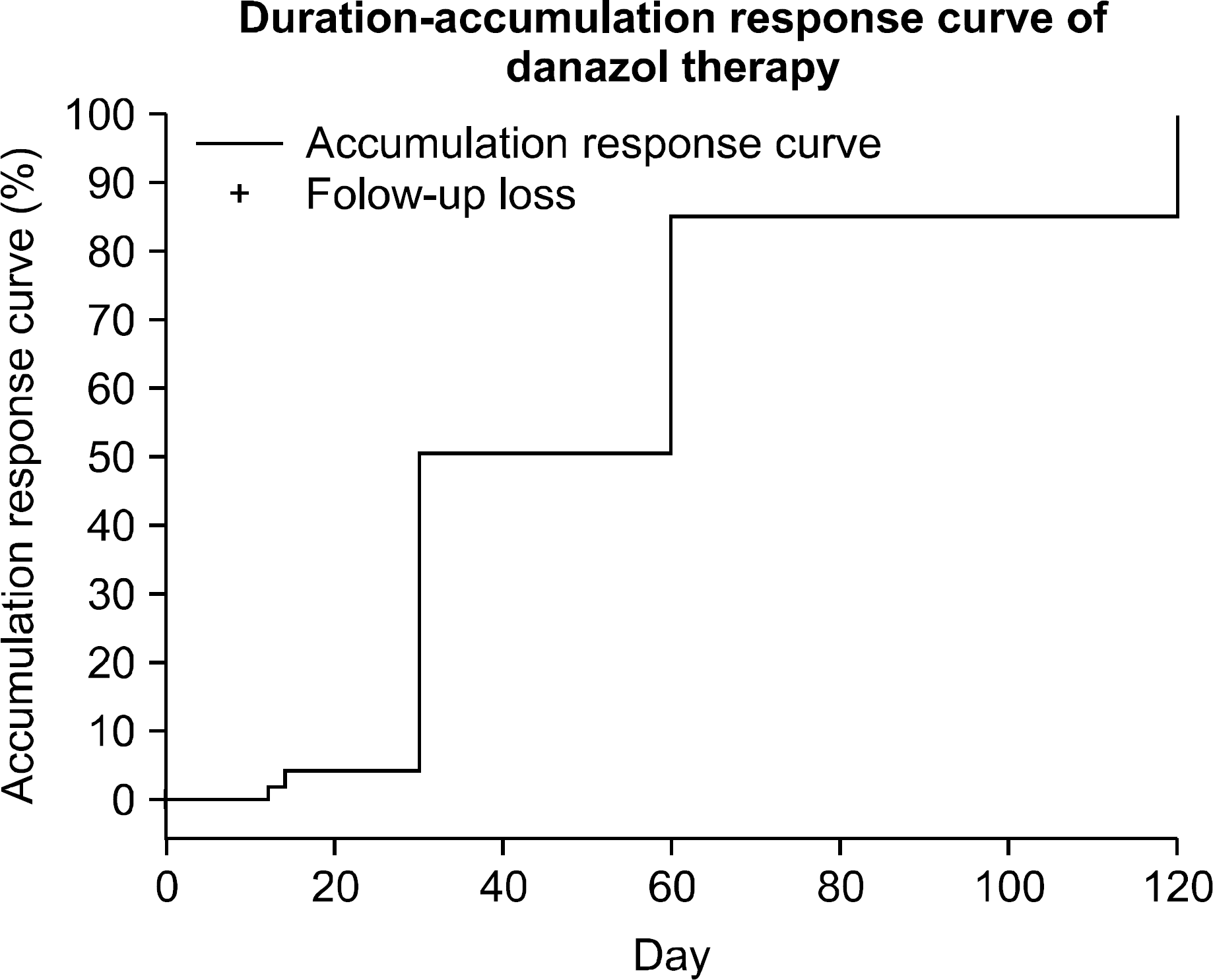
Table 1.
Clinical characteristics of the 28 patients
Table 2.
Factors influencing the response to danazol




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download