Abstract
Background:
We retrospectively evaluated the treatment outcomes and toxicities of Hodgkin's disease (HD) patients treated by ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine) combination chemotherapy, and compared them with those of a historical group treated with a CVPP (cyclophosphamide, vinblastine, procarbazine, and prednisone) regimen.
Methods:
The medical records of patients who had been diagnosed with HD histologically and treated by either ABVD or CVPP from 1997 to 2006 at the Korea University Medical Center were retrospectively reviewed.
Results:
Thirty patients were eligible. Nineteen patients received ABVD and eleven patients were treated with CVPP. The response rates for ABVD and CVPP were 84.21% and 54.55%, respectively. Median overall survival was 43.17 months for ABVD and 43.27 months for CVPP (P=.570). Median event-free survival was 39.03 months for ABVD and 16.73 months for CVPP (P=.088). There was no significant difference in median survival or in event-free survival between the two regimens. Hematologic toxicities were significantly more common in the CVPP group than in the ABVD group. Grade 3 or 4 neutropenia was observed in 72.72% of the CVPP group and in 36.84% of the ABVD group (P=.050).
Go to : 
REFERENCES
1). Devita VT Jr., Serpick AA., Carbone PP. Combination chemotherapy in the treatment of advanced Hodgkin's disease. Ann Intern Med. 1970. 73:881–95.
2). Bakemeier RF., Anderson JR., Costello W, et al. BCVPP chemotherapy for advanced Hodgkin's disease: evidence for greater duration of complete remission, greater survival, and less toxicity than with a MOPP regimen. Ann Intern Med. 1984. 101:447–56.

3). Rosenberg SA., Kaplan HS. The evolution and summary results of the Stanford randomized clinical trials of the management of Hodgkin's disease: 1962-1984. Int J Radiat Oncol Biol Phys. 1985. 11:5–22.

4). Bonadonna G., Santoro A. ABVD chemotherapy in the treatment of Hodgkin's disease. Cancer Treat Rep. 1982. 66:439–49.

5). Santoro A., Bonfante V., Bonadonna G. Salvage chemotherapy with ABVD in MOPP-resistant Hodgkin's disease. Ann Intern Med. 1982. 96:139–43.

6). Josting A., Franklin J., May M, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin's lymphoma registered in the database of German Hodgkin's Lymphoma Study Group. J Clin Oncol. 2002. 20:221–30.
7). Bonfante V., Santoro A., Viviani S, et al. Outcome of patients with Hodgkin's disease failing after primary MOPP-ABVD. J Clin Oncol. 1997. 15:528–34.

8). Duggan DB., Petroni GR., Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin's disease: report of an intergroup trial. J Clin Oncol. 2003. 21:607–14.

9). Johnson PW., Radford JA., Cullen MH, et al. Comparison of ABVD and alternating or hybrid multidrug regimens for the treatment of Hodgkin's lymphoma: results of the United Kingdom Lymphoma Group LY09 Trial (ISRCTN97144519). J Clin Oncol. 2005. 23:9208–18.
10). Hahn JS., Ko YW., Min YH, et al. Statistical analysis of malignant lymphoma in Korea. Korean J He-matol. 1995. 30:197–214.
11). Kim HT., Im YH., Suh CI, et al. Malignant lymphoma in Korea. J Korean Cancer Assoc. 1992. 24:92–101.
12). Pavlovsky S., Schvartzman E., Lastiri F, et al. Randomized trial of CVPP for three versus six cycles in favorable-prognosis and CVPP versus AOPE plus radiotherapy in intermediate-prognosis untreated Hodgkin's disease. J Clin Oncol. 1997. 15:2652–8.

13). Lister TA., Crowther D., Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol. 1989. 7:1630–6.

14). Longo DL., Young RC., Wesley M, et al. Twenty years of MOPP therapy for Hodgkin's disease. J Clin Oncol. 1986. 4:1295–306.

15). McElwain TJ., Toy J., Smith E., Peckham MJ., Austin DE. A combination of chlorambucil, vinblastine, procarbazine and prednisolone for treatment of Hodgkin's disease. Br J Cancer. 1977. 36:276–80.

16). Hehn ST., Miller TP. What is the treatment of choice for advanced-stage Hodgkin's lymphoma: ABVD, Stanford V, or BEACOPP. Curr Hematol Rep. 2004. 3:17–26.
18). Kim KW., Chang YB., Kim MC., Son CH., Park SJ., Chung DG. Clinical observation on 55 cases of Hodgkin's disease. Korean J Hematol. 1982. 17:35–44.
19). Ryoo BY., Kim HG., Kim TY, et al. C-MOPP/ABV (cyclophosphamide, vincristine, procarbazine, prednisolone/doxorubicin, bleomycin, vinblastine) hybrid chemotherapy for previously untreated advanced Hodgkin's disease. Korean J Hematol. 1998. 33:54–64.
20). Ahn JS., Lee KS., Lee JT, et al. COPP/ABV hybrid chemotherapy in patients with Hodgkin's disease. J Korean Cancer Assoc. 1998. 30:818–26.
21). Cheong JW., Park SY., Roh JK., Suh CO., Han JS. Treatment of Hodgkin's disease: a twenty-year follow-up of patients at a center in Korea. Yonsei Med J. 2006. 47:455–65.

22). Canellos GP. Can MOPP be replaced in the treatment of advanced Hodgkin's disease? Semin Oncol. 1990. 17:2–6.
23). Canellos GP., Anderson JR., Propert KJ, et al. Chemotherapy of advanced Hodgkin's disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992. 327:1478–84.

24). Landgren O., Algernon C., Axdorph U, et al. Hodgkin's lymphoma in elderly with special reference to type and intensity of chemotherapy in relation to prognosis. Haematologica. 2003. 88:438–44.
Go to : 
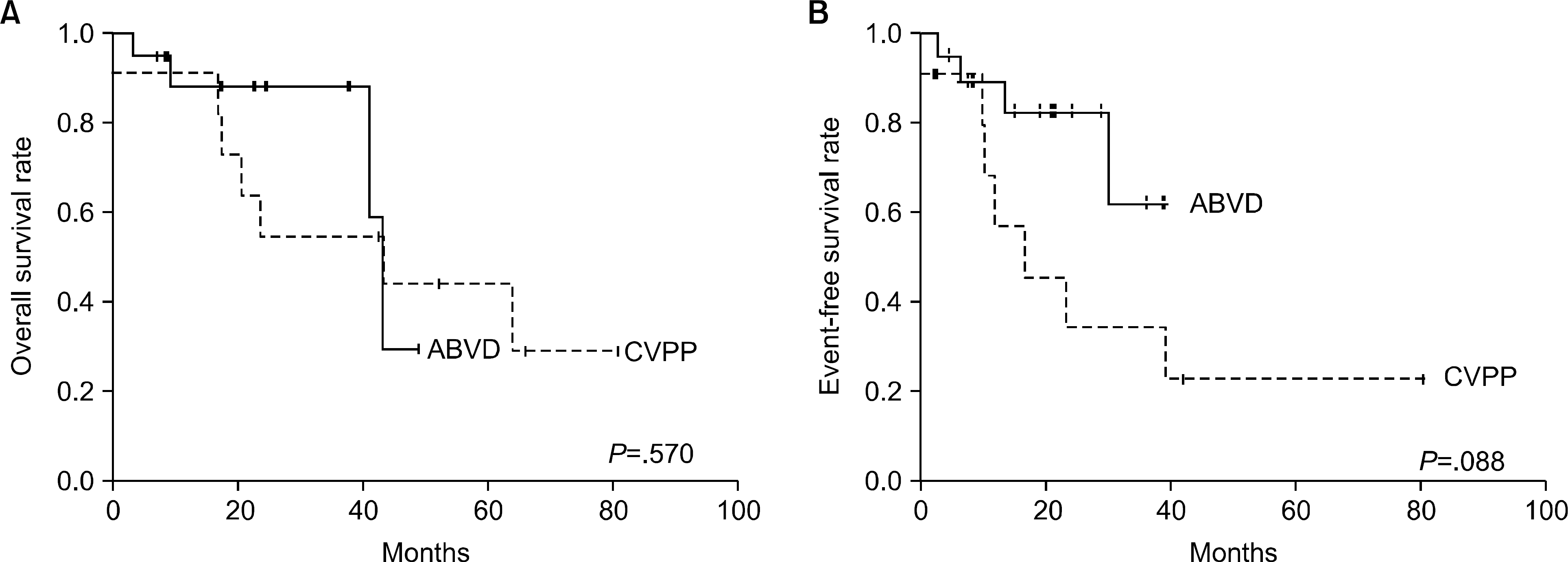 | Fig. 1Defined outcomes according to the regimens. Overall survival rate (A) and event free survival rate (B). |
Table 1.
Baseline characteristics of the 30 eligible patients
Abbreviations: CVPP, cyclophosphamide, vinblastine, procarbazine, and prednisone; ABVD, adriamycin, bleomycin, vinblastine, and dacarbazine; LDH, lactate dehydrogenase; ESR, erythrocyte sedimentation rate; LP, lymphocyte predominance; NS, nodular sclerosis; MC, mixed cellularity; UC, unclassifiable.
Table 2.
Respnse data by treatment group
| Response | CVPP | ABVD | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Complete response | 2 | 18.2 | 9 | 47.4 |
| Partial response | 4 | 36.3 | 7 | 36.8 |
| Stable disease | 3 | 27.3 | 1 | 5.3 |
| Progressive disease | 2 | 18.2 | 2 | 10.5 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download