Abstract
Background:
Autologous peripheral hematopoietic stem cell transplantation (APBSCT) has been widely used to treat various types of hematological disorders, metabolic diseases and congenital immunodeficiency. Hematopoietic recovery is important because prolonged duration of neutropenia and thrombocytopenia is associated with a higher risk of infection, bleeding and treatment related mortality. Many investigators have studied the factors that affect hematopoietic recovery after stem cell transplantation.
Methods:
We retrospectively investigated the factors influencing hematopoietic engraftment in 112 patients with hematological malignancies and solid tumors who received APBSCT. We evaluated the gender, age, CD34+ cell number, conditioning regimens, and the type of tumor and their association with neutrophil and platelet engraftment.
Results:
Post-transplant neutrophil engraftment (>500/μL) required a median of 11 days (range 6∼50) and platelet engraftment 12 (range 1∼78) days (>20,000/μL). The univariate analysis showed that the factors that positively affected hematopoietic recovery were: the type of conditioning regimens such as BEAM (BCNU, etoposide, cytosine arabinoside, melphalan) and BEAC (BCNU, etoposide, cytosine arabinoside, cyclophosphamide) versus BC (busulfan, cyclophosphamide), the CD34+ cell number and the disease diagnosis such as multiple myeloma versus acute myelogenous leukemia. The multivariate analysis showed only the CD34+ cell number (5∼10×106/kg) to be significantly associated with early neutrophil and platelet engraftment (P<.001).
REFERENCES
2). Bearman SI., Appelbaum FR., Buckner CD, et al. Regimen related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988. 6:1562–8.
3). Singhal S., Powles R., Kulkarni S, et al. Comparison of marrow and blood cell yields from the same donors in a double-blind, randomized study of allogeneic marrow vs. blood stem cell transplantation. Bone Marrow Transplant. 2000. 25:501–5.

4). Shpall EJ., Champlin R., Glaspy JA, et al. Effect of CD34+ peripheral blood progenitor cell dose on hematopoietic recovery. Biol Blood Marrow Transplant. 1998. 4:84–92.

5). Siena S., Bregni M., Brando B, et al. Flow cytometry for clinical estimation of circulating hematopoietic progenitors for autologous transplantation in cancer patients. Blood. 1991. 77:400–9.

6). Lee J., Lee MH., Park KW, et al. Influential factors for the collection of peripheral blood stem cells and engraftment in acute myeloid leukemia patients in first complete remission. Int J Hematol. 2005. 81:258–63.

7). Chae YS., Jeon SB., Sung WJ, et al. Clinical outcomes according to transplanted CD34+ cell dose in allogeneic peripheral blood stem cell transplantation. Korean J Hematol. 2003. 38:24–31.
8). Woo HY., Kim HR., Seong KW, et al. Correlation between progenitor cell dose and the rate of engraftment in autologous peripheral blood. Korean J Blood Transfus. 2000. 11:35–47.
9). Neben S., Hellman S., Montgomery M., Ferrara J., Mauch P. Hematopoietic stem cell deficit of transplanted bone marrow previously exposed to cytotoxic agents. Exp Hematol. 1993. 21:156–62.
10). Carral A., de la Rubia J., Martin G, et al. Factors influencing hematopoietic recovery after autologous blood stem cell transplantation in patients with acute myeloblastic leukemia and with non-myeloid malignancies. Bone Marrow Transplant. 2002. 29:825–32.

11). Ergene U., Cagirgan S., Pehlivan M., Yilmaz M., Tombuloglu M. Factors influencing engraftment in autologous peripheral hematopoietic stem cell transplantation (PBSCT). Transfus Apher Sci. 2007. 36:23–9.
12). Trenschel R., Bernier M., Delforge A, et al. Myeloid and lymphoid recovery following bone marrow transplantation: A comparative study between related, unrelated bone marrow and allogeneic stem cell transplantation. Leuk Lymphoma. 1998. 30:325–52.
13). Singhal S., Powles R., Treleaven J, et al. A low CD34+ cell dose results in higher mortality and poorer survival after blood or marrow stem cell transplantation from HLA-identical siblings: should 2×106 CD34+ cells/kg be considered the minimum threshold? Bone Marrow Transplant. 2000. 26:489–96.
14). Perez-Simon JA., Caballero MD., Corral M, et al. Minimal number of circulating CD34+ cells to ensure successful leukapheresis and engraftment in autologous peripheral blood progenitor cell transplantation. Transfusion. 1998. 38:385–91.

15). Zubair AC., Zahrieh D., Daley H, et al. Engraftment of autologous and allogeneic marrow HPCs after myeloablative therapy. Transfusion. 2004. 44:253–61.

16). Siena S., Schiavo R., Pedrazzoli P., Carlo-Stella C. Therapeutic relevance of CD34+ cell dose in blood cell transplantation for cancer therapy. J Clin Oncol. 2000. 18:1360–77.

17). Weaver CH., Hazelton B., Birch R, et al. Analysis of engraftment kinetics as a function of the CD34+ content of peripheral blood progenitor cell collection in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995. 86:3961–9.
18). Edenfield WJ., Moores LK., Goodwin G., Lee N. An engraftment syndrome in autologous stem cell transplantation related to mononuclear cell dose. Bone Marrow Transplant. 2000. 25:405–9.

19). Chen CH., Lin W., Shye S., Kibler R, et al. Automated enumeration of CD34+ cells in peripheral blood and bone marrow. J Hematother. 1994. 3:3–13.
Fig. 1
Analysis of the correlation between CD34+ cell dose and engraftment in Auto PBSCT illustrate that infused CD34+ cell/kg is significantly correlated with the time to neutrophil and platelet engraftment.
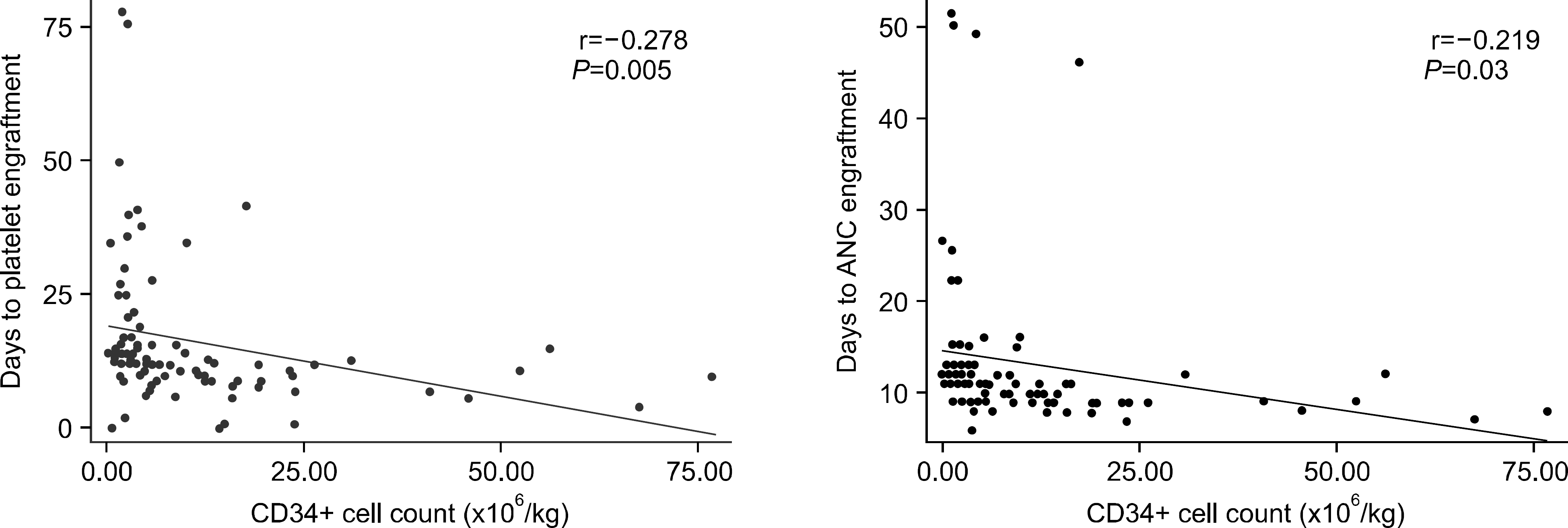
Fig. 2
Kaplan-Meier cumulative probability curves illustrate CD34+ cell number (>5.0×106/kg) is significantly associated with neutrophil and platelet engraftment in APBSCT (P<.001).
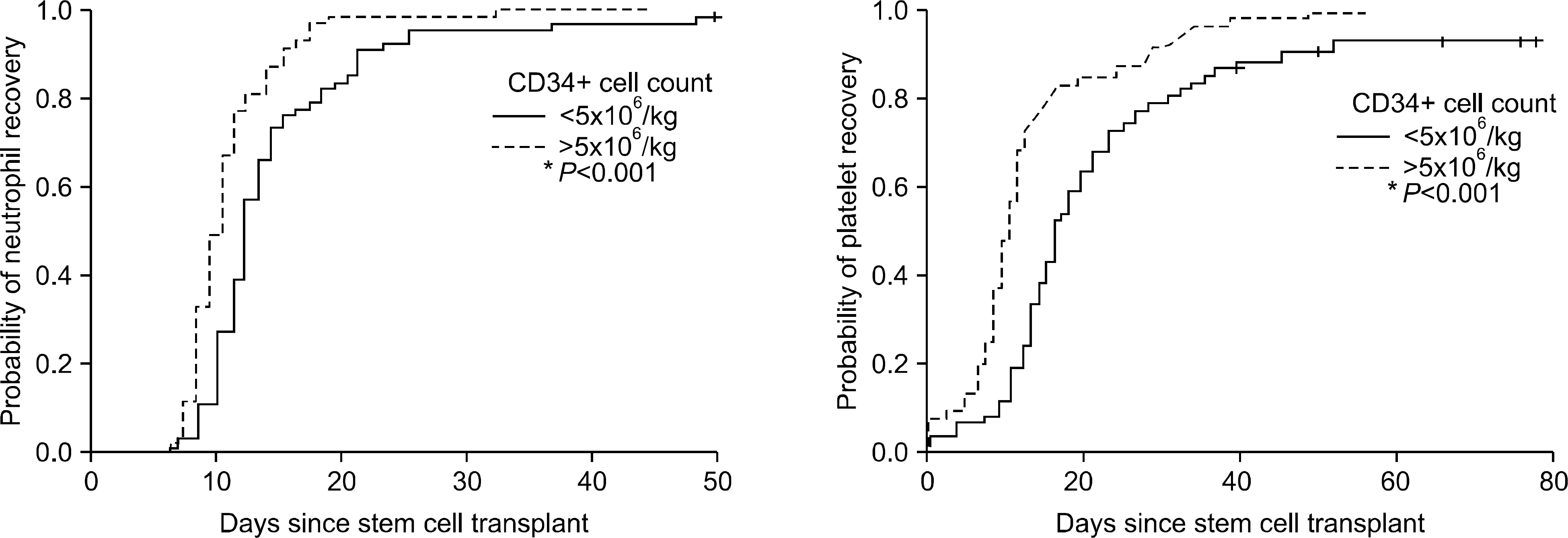
Table 1.
Patient characteristics
Table 2.
Correlations between progenitor cell dose and hematopoietic engraftment time
Table 3.
Conditioning regimens and hematopoietic engraftment
∗Statistical significance test done by log rank test P value<0.05. Abbreviations: BEAM, BCNU, etoposide, cytosine arabinoside, melphalan; BEAC, BCNU, etoposide, cytosine arabinoside cyclophosphamide; BUCY, busulfan, cyclophosphamide; ICE, ifosphamide, carboplatin, etoposide; TMJ, thiotepa, mitoxan trone, carboplatin.
Table 4.
Diseases and hematopoietic engraftment
Table 5.
Other influential factors for hematopoietic engraftment




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download