Abstract
Human parvovirus B19 infection could be manifested as pure red cell aplasia or chronic anemia in immunocompromised host. The patient was 35-year-old female who had been diagnosed as non-Hodgkin lymphoma, peripheral T-cell unspecified type and had been performed chemotherapy. She complained headache and dizziness that was found to a marked drop in hemoglobin (3.2g/dL). A bone marrow aspiration revealed findings consistent with erythroid hypoplasia with maturation arrest. Serum parvovirus B19 PCR and anti parvovirus B19 IgM were positive. After immunoglobulin therapy, it was leading to a marked increase in reticulocyte count and corresponding rise in hemoglobin. To our knowledge, this is the first report to use immunoglobulin in an adult cancer patient with pure red-cell aplasia. Human parvovirus B19 infection should be considered in immunocompromised cancer patients with red cell aplasia and early use of immunoglobulins would be helpful in resolution of anemia and not to delay planned chemotherapy.
REFERENCES
1). Brown KE., Anderson SM., Young NS. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science. 1993. 262:114–7.

2). Anderson LJ. Human parvovirus. J Infect Dis. 1990. 161:603–8.
3). Pamidi S., Friedman K., Kampalath B., Eshoa C., Hariharan S. Human parvovirus B19 infection presenting as persistent anemia in renal transplant recipients. Transplantation. 2000. 69:2ㅌ. 666–9.

4). Frickhofen N., Abkowitz JL., Safford M, et al. Persistent B19 parvovirus infection in patients infected human immunodeficiency virus type 1 (HIV-1): a treatable cause of anemia in AIDS. Ann Intern Med. 1990. 113:926–33.
5). Koduri PR., Kumapley R., Valladares J., Teter C. Chronic pure red cell aplasia caused by parvovirus B19 in AIDS: use of intravenous immunoglobulin-a report of eight patients. Am J Hematol. 1999. 61:16–20.

6). Isobe Y., Sugimoto K., Shiraki Y., Nishitani M., Koike K., Oshimi K. Successful high-titer immunoglobulin therapy for persistent parvovirus B19 infection in a lymphoma patient treated with rituximab-combined chemotherapy. Am J Hematol. 2004. 77:370–3.

7). Song KW., Mollee P., Patterson B., Brien W., Crump M. Pure red cell aplasia due to parvovirus following treatment with CHOP and rituximab for B-cell lymphoma. Br J Haematol. 2002. 119:125–7.

8). Ghazal H. Successful treatment of pure red cell aplasia with rituximab in patients with chronic lymphocytic leukemia. Blood. 2002. 99:1092–4.

9). Batlle M., Ribera JM., Oriol A., Plensa E., Milla F., Feliu E. Successful response to rituximab in a patient with pure red cell aplasia complicating chronic lymphocytic leukemia. Br J Haematol. 2002. 118:1192–3.
10). Lee YJ., So BJ., Chae KM., Jeong BH. A case of pure red cell aplasia due to parvovirus B19 in renal transplantation. Korean J Hematol. 1999. 34:646–50.
11). Park AJ., Lim YA., Jeon HS, et al. A case of transient aplastic crisis induced by human parvovirus Bl9 in hereditary spherocytosis. Korean J Hematol. 1996. 31:313–8.
12). Jung HJ., Kim EC., Cho HI, et al. Chronic hypoplastic anemia caused by human parvovirus B19 in a patient with non-Hodgkin's lymphoma. Korean J Lab Med. 1993. 13:587–94.
13). Brown KE. Parvovirus B19. Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. vol. 2. 6th ed.Philadelphia, USA: Elsevier Churchill Living-stone;2005. p. 1891–8.
14). Koch WC., Adler SP. Detection of human parvovirus B19 DNA by using the polymerase chain reaction. J Clin Microbiol. 1990. 28:65–9.

15). Brown KE., Hibbs JR., Gallinella G, et al. Resistance to parvovirus B19 infection due to lack of virus receptor (erythrocyte P antigen). N Engl J Med. 1994. 330:1192–6.

16). Erdman DD. Human parvovirus B19: laboratory diagnosis. Anderson LJ, Young NS, editors. Human parvovirus B19. Vol. 20. Monographs in virology. Basel, Switzerland: Karger;1997. p. 93–104.

Fig. 1
Bone marrow aspiration smear, Wright stain ×1,000 (A) and bone marrow biopsy, H & E stain ×400 (B). Erythroid precursors were markedly decreased in bone marrow aspiration and M:E ratio was 72:1. But, granulocytic precursors were normal in number and in distribution. Bone marrow biopsy shows normocellular and the cellularity was 50%. Megakaryocytes and granulocytic precursors were normal in number and distribution but erythroid precursors were hardly observed.

Fig. 2
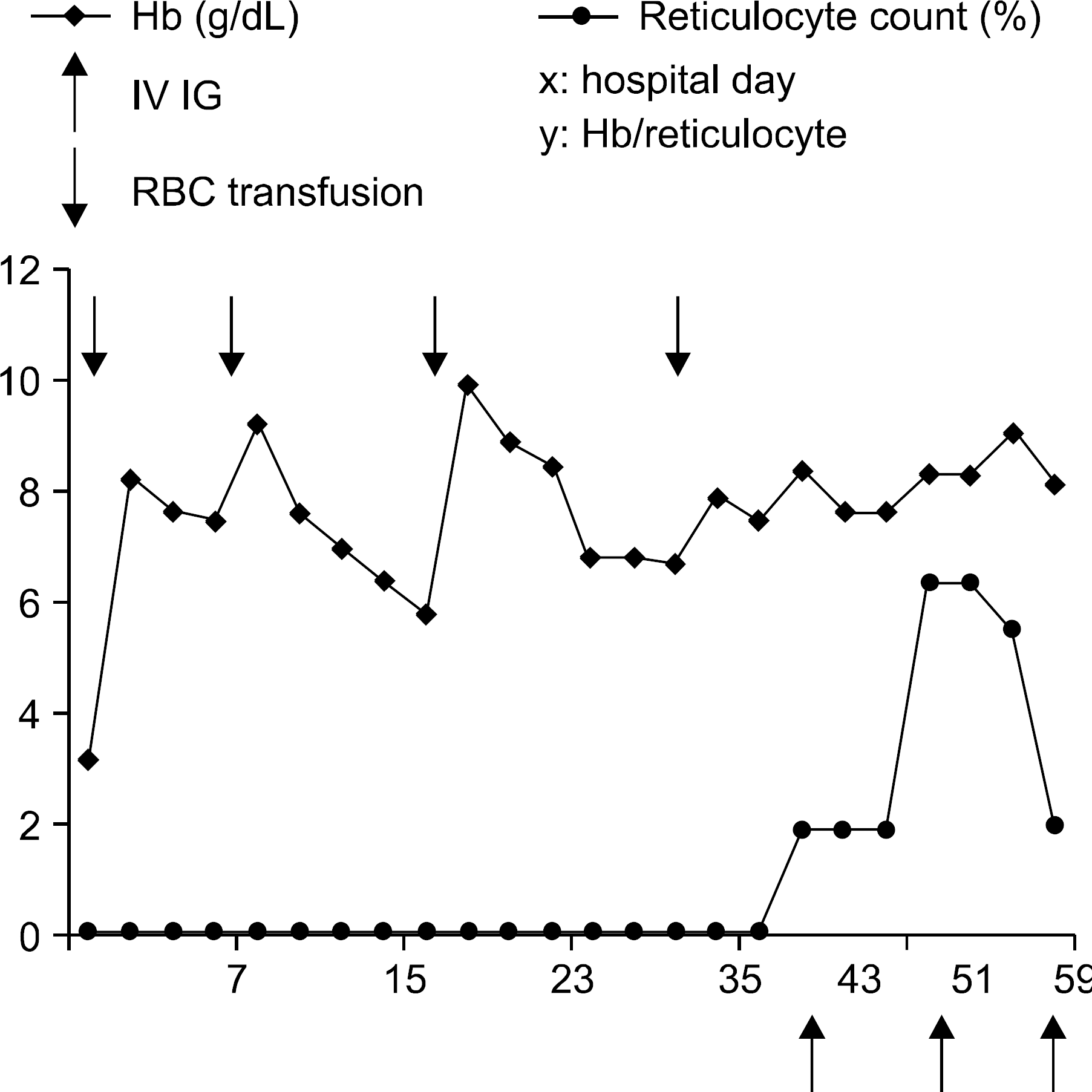
Change of hemoglobin and reticulocyte count during hospital course. Despite of RBC transfusion in total 4 times, hemoglobin level was 6.7g/dL and reticulocyte count was 0.1%. At 39th hospital day (HD), we provided intravenous immunoglobulin (400mg/kg weekly). At 43th HD, we observed a marked increase in reticulocyte count and corresponding rise in hemoglobin. At 50th HD, hemoglobin level recovered to 9.1g/dL and reticulocyte count rose to 6.4% without transfusion.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download