Abstract
Background:
The presence of FLT3 internal tandem dupulication (FLT3/ITD) in patients with acute myeloid leukemia (AML) with normal karyotype was investigated in order to evaluate its clinical and prognostic significance.
Methods:
The FLT3/ITD was studied by PCR assay in bone marrow samples obtained from 123 patients at diagnosis. Ninety patients who received intensive induction chemotherapy were evaluated.
Results:
Of total 123 patients, forty-seven (38.2%) demonstrated the aberrant FLT3/ITD. Patients with FLT3/ITD had significantly higher leukocyte counts at presentation than did patients without FLT3/ITD (P=0.04). By multivariate analysis, the FLT3/ITD was an independent prognostic factor of leukemic-free survival (LFS) (P=0.01) in AML patients with normal karyotype.
Go to : 
REFERENCES
1). Rosnet O., Schiff C., Pebusque MJ, et al. Human FLT3/FLK2 gene: cDNA cloning and expression in hematopoietic cells. Blood. 1993. 82:1110–9.

2). Small D., Levenstein M., Kim E, et al. STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc Natl Acad Sci USA. 1994. 91:459–63.

3). Abu-Duhier FM., Goodeve AC., Wilson GA, et al. FLT3 internal tandem duplication mutations in adult acute myeloid leukemia define a high-risk group. Br J Haematol. 2000. 111:190–5.
4). Kottaridis PD., Gale RE., Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001. 98:1752–9.

5). Kiyoi H., Towatari M., Yokota S, et al. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia. 1998. 12:1333–7.

6). Hayakawa F., Towatari M., Kiyoi H, et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene. 2000. 19:624–31.

7). Tse KF., Mukherjee G., Small D. Constitutive activation of FLT3 stimulates multiple intracellular signal transducers and results in transformation. Leukemia. 2000. 14:1766–76.

8). Ciolli S., Vannucchi AM., Leoni F, et al. Internal tandem duplications of Flt3 gene (FLT3/ITDs) predicts a poor post-remission outcome in adult patients with acute non-promyelocytic leukemia. Leuk Lymphoma. 2004. 45:73–8.
9). Kainz B., Heintel D., Marculescu R, et al. Variable prognostic value of FLT3 internal tandem duplications in patients with de novo AML and a normal karyotype, t(15;17), t(8;21) or inv(16). Hematol J. 2002. 3:283–9.

10). Beran M., Luthra R., Kantarjian H., Estey E. FLT3 mutation and response to intensive chemotherapy in young adult and elderly patients with normal karyotype. Leuk Res. 2004. 28:547–50.

11). Chillon MC., Fernandez C., Garcia-Sanz R, et al. FLT3-activating mutations are associated with poor prognostic features in AML at diagnosis but they are not an independent prognostic factor. Hematol J. 2004. 5:239–46.

12). Cheson BD., Cassileth PA., Head DR, et al. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990. 8:813–9.

13). Grimwade D., Walker H., Harrison G, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001. 98:1312–20.

14). Slovak ML., Kopecky KJ., Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a southwest oncology group/eastern cooperative oncology group study. Blood. 2000. 96:4075–83.

15). Birg F., Rosnet O., Carbuccia N., Birnbaum D. The expression of FMS, KIT and FLT3 in hematopoietic malignancies. Leuk Lymphoma. 1994. 13:223–7.

16). Shurin MR., Esche C., Lotze MT. FLT3: receptor and ligand. Biology and potential clinical application. Cytokine Growth Factor Rev. 1998. 9:37–48.

17). McKenna HJ., Stocking KL., Miller RE, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000. 95:3489–97.

18). Lisovsky M., Estrov Z., Zhang X, et al. Flt3 ligand stimulates proliferation and inhibits apoptosis of acute myeloid leukemia cells: regulation of Bcl-2 and Bax. Blood. 1996. 88:3987–97.

19). Fenski R., Flesch K., Serve S, et al. Constitutive activation of FLT3 in acute myeloid leukaemia and its consequences for growth of 32D cells. Br J Hae-matol. 2000. 108:322–30.

20). Mizuki M., Fenski R., Halfter H, et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood. 2000. 96:3907–14.

21). Kiyoi H., Naoe T., Nakano Y, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999. 93:3074–80.
22). Ozeki K., Kiyoi H., Hirose Y, et al. Biologic and clinical significance of the FLT3 transcript level in acute myeloid leukemia. Blood. 2004. 103:1901–8.

23). Frohling S., Schlenk RF., Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002. 100:4372–80.
24). Au WY., Fung A., Chim CS, et al. FLT-3 aberrations in acute promyelocytic leukaemia: clinicopathological associations and prognostic impact. Br J Haematol. 2004. 125:463–9.

25). Whitman SP., Archer KJ., Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res. 2001. 61:7233–9.
Go to : 
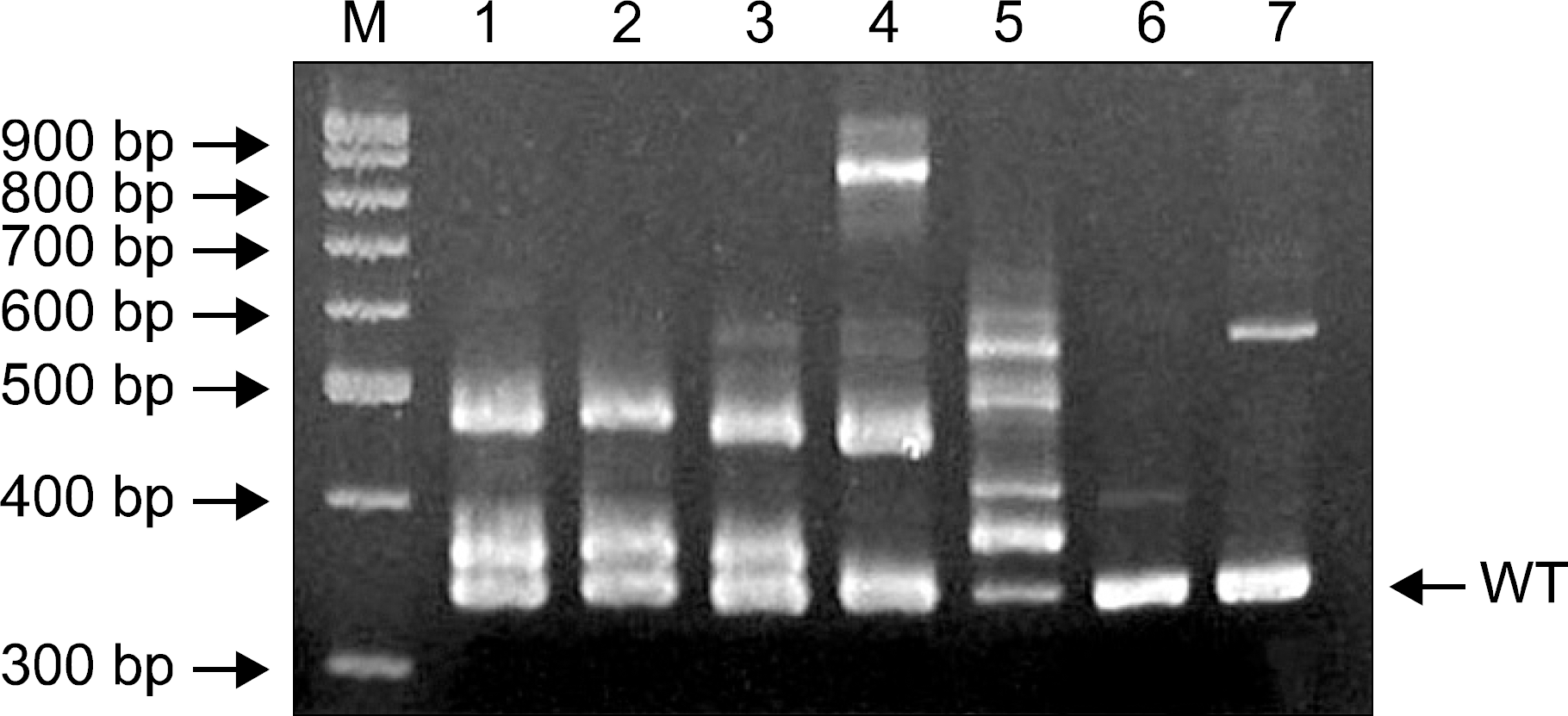 | Fig. 1PCR products of the FLT3/ITD, analyzed by gel electrophoresis. Reverse view of ethidium-bromide-stained 6% polyacrylamide gel. FLT3/ITD was detected as extra-bands in addition to normal band of 328 bp. Lanes 1 to 7 show PCR results in seven patients with FLT3/ITD. M: molecular weight markers, WT: wild type. |
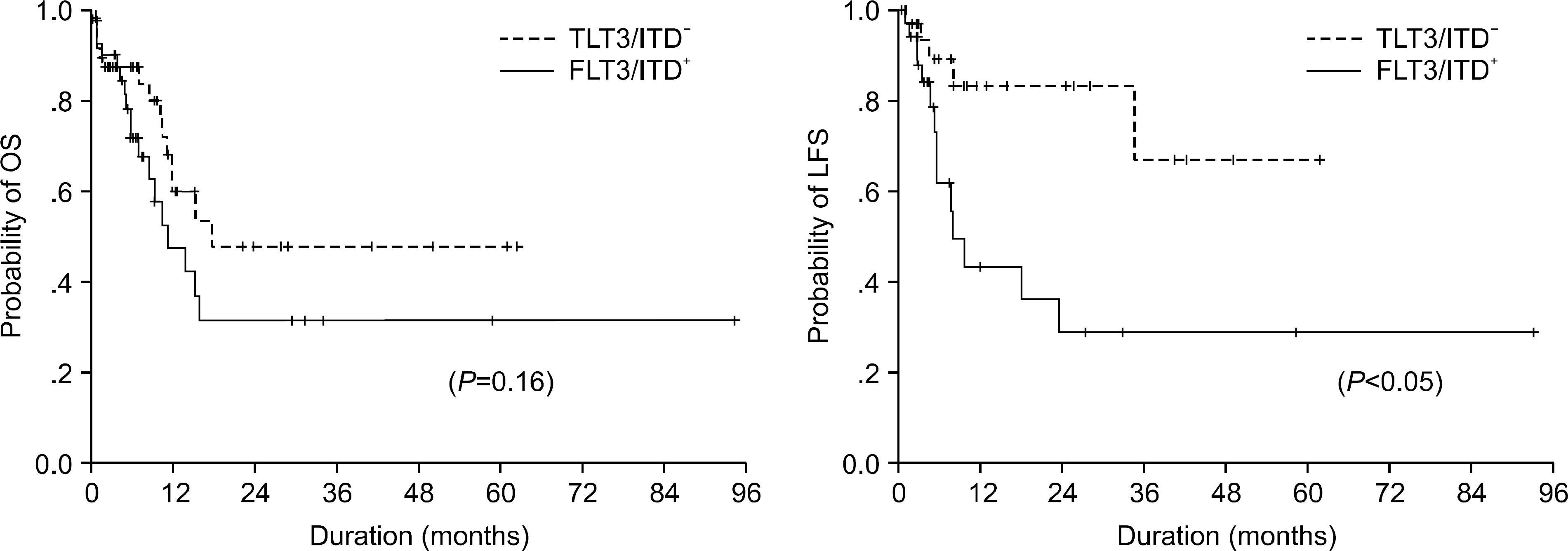 | Fig. 2Overall survival (OS) (upper panel) and leukemic-free survival (LFS) (lower panel) of AML patients with normal karyotype before transplantation. A trend for longer survival was observed in patients without FLT3/ITD compared with those with FLT3/ITD, but there was no statistical significance. A significant difference in LFS was found between FLT3/ITD-positive and FLT3/ITD-negative patients (OS: P=0.16; LFS: P=0.01 by the log rank test with pooled over strata). |
Table 1.
Clinical and biological features in 123 acute myeloid leukemia (AML) patients with normal karyotype according to FLT3/ITD expression
Table 2.
Multivariate analysis of prognostic factors for LFS




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download