Abstract
Background:
The Polycomb-group gene Bmi-1 is known to be a molecular regulator of self-renewal of normal and leukemic stem cells and be involved in various aspects of cellular proliferation, differentiation, and survival.
Methods:
This study evaluated the effects of overexpression of Bmi-1 on human cord blood CD34+ cells. Bmi-1 was introduced into CD34+ cells through lentivirus transduction. Bmi-1 expressing CD34+ cells were applied to colony forming assay, stromal co-culture, and cytokine-stimulatied culture.
Results:
Ectopic expression of Bmi-1 resulted in the increased number of erythroid colonies in primary and secondary colony forming assay in an erythropoietin dependent manner. In stromal co-culture, Bmi-1-expressing postnatal hematopoietic stem cells seemed to lose the ability of self-renewal, as determined by week 5 cobblestone area-forming cell assay and by week 5 secondary colony assay. In cytokine-stimulated suspension culture of Bmi-1-transduced CD34+ cells, we observed increased erythropoiesis marked by Glycophorin A expression.
REFERENCES
1). Lund AH., van Lohuizen M. Polycomb complexes and silencing mechanisms. Curr Opin Cell Biol. 2004. 16:239–46.

3). Molofsky AV., He S., Bydon M., Morrison SJ., Pardal R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005. 19:1432–7.

4). Molofsky AV., Pardal R., Iwashita T., Park IK., Clarke MF., Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003. 425:962–7.

5). Christopher FA., Dimos JT., Ivanova NB., Lowry N., Lemischka IR., Temple S. shRNA Knockdown of Bmi-1 Reveals a Critical Role for p21-Rb Pathway in NSC Self-Renewal during Development. Cell Stem Cell. 2007. 1:12.

6). Park IK., Qian D., Kiel M, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003. 423:302–5.

7). Iwama A., Oguro H., Negishi M, et al. Enhanced self-renewal of hematopoietic stem cells mediated by the polycomb gene product Bmi-1. Immunity. 2004. 21:843–51.

8). Lessard J., Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003. 423:255–60.

9). Lessard J., Baban S., Sauvageau G. Stage-specific expression of polycomb group genes in human bone marrow cells. Blood. 1998. 91:1216–24.

10). Hemmati HD., Nakano I., Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA. 2003. 100:15178–83.

11). Mihic-Probst D., Kuster A., Kilgus S, et al. Consistent expression of the stem cell renewal factor BMI-1 in primary and metastatic melanoma. Int J Cancer. 2007. 121:1764–70.

12). Glinsky GV., Berezovska O., Glinskii AB. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest. 2005. 115:1503–21.

13). Jacobs JJ., Kieboom K., Marino S., DePinho RA., van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999. 397:164–8.

14). Jacobs JJ., Scheijen B., Voncken JW., Kieboom K., Berns A., van Lohuizen M. Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes Dev. 1999. 13:2678–90.

15). Zhang P., Iwasaki-Arai J., Iwasaki H, et al. Enhancement of hematopoietic stem cell repopulating capacity and self-renewal in the absence of the transcription factor C/EBP alpha. Immunity. 2004. 21:853–63.
16). Oneal PA., Gantt NM., Schwartz JD, et al. Fetal hemoglobin silencing in humans. Blood. 2006. 108:2081–6.

18). Walkley CR., Shea JM., Sims NA., Purton LE., Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow micro-environment. Cell. 2007. 129:1081–95.
Fig. 1
Construction of FUEG and FUEG-Bmi-1 lentivirus and detection of elevated Bmi-1 expression in transduced 293T and human cord blood (CB) CD34+ cells. (A) A schematic image of FUEG and FUEG-Bmi-1 lentivirus used in this study. (B) Immunoblot of whole-cell protein extracts from transduced 293T and GFP+FACS-sorted human CB-derived CD34+ cells with anti-Bmi-1 and anti-Actin antibodies.
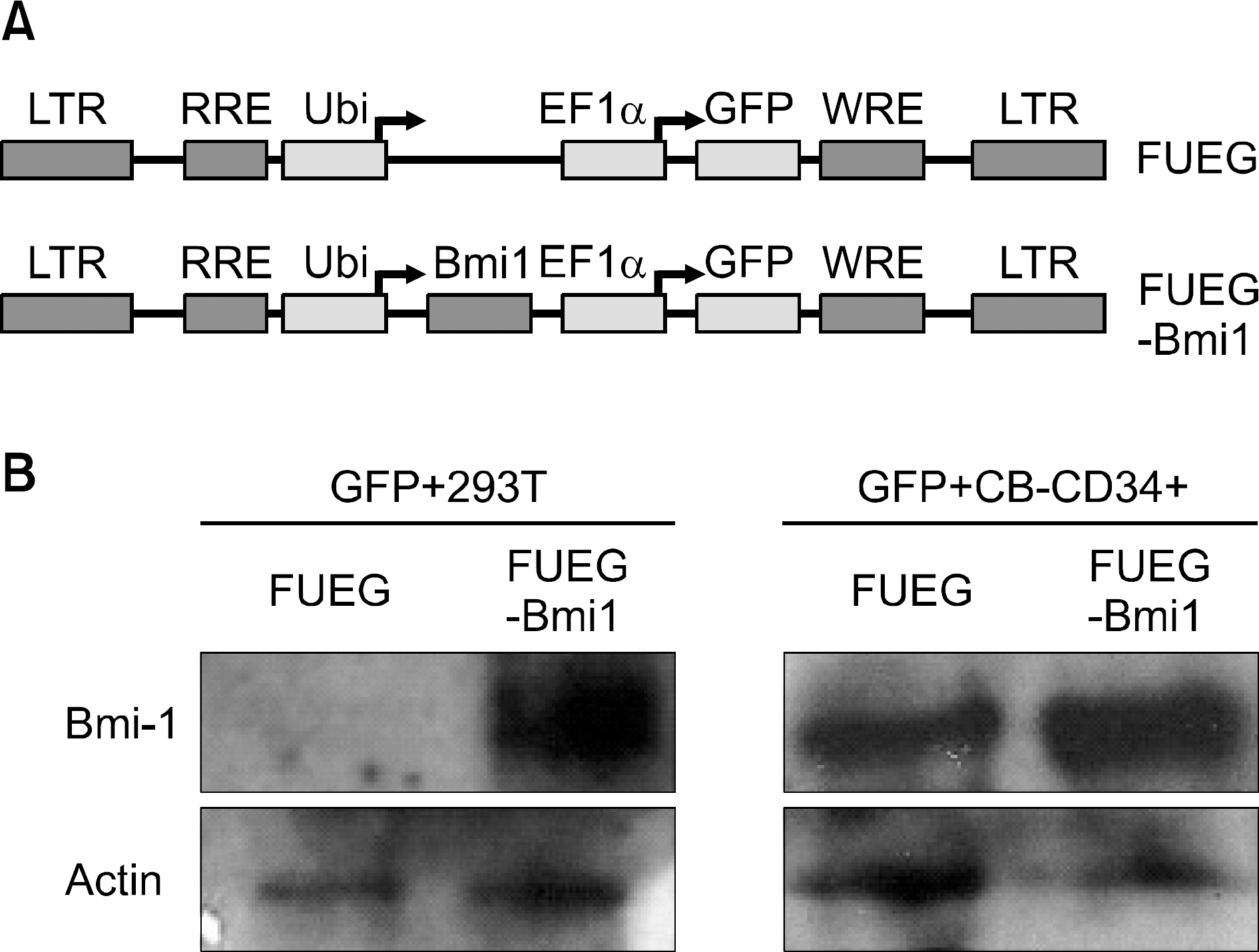
Fig. 2
Ectopic Bmi-1 expression in CB CD34+ cells enhances the formation of both primary and secondary erythroid colonies. Numbers of erythroid colonies in primary (A) and secondary (B) The number of colony-forming cells (CFCs) was significantly increased in FUEG-Bmi-1 group (∗P<0.05, Error bar is not shown). (C) Representative images for bright field (BF) and GFP expression (GFP) of each type of colonies from primary CFC assay in FUEG-Bmi-1 group. (D) Representative images for GFP expression of CFU-E and cytospin images of the cells obtained from secondary colonies in FUEG-Bmi-1 group.
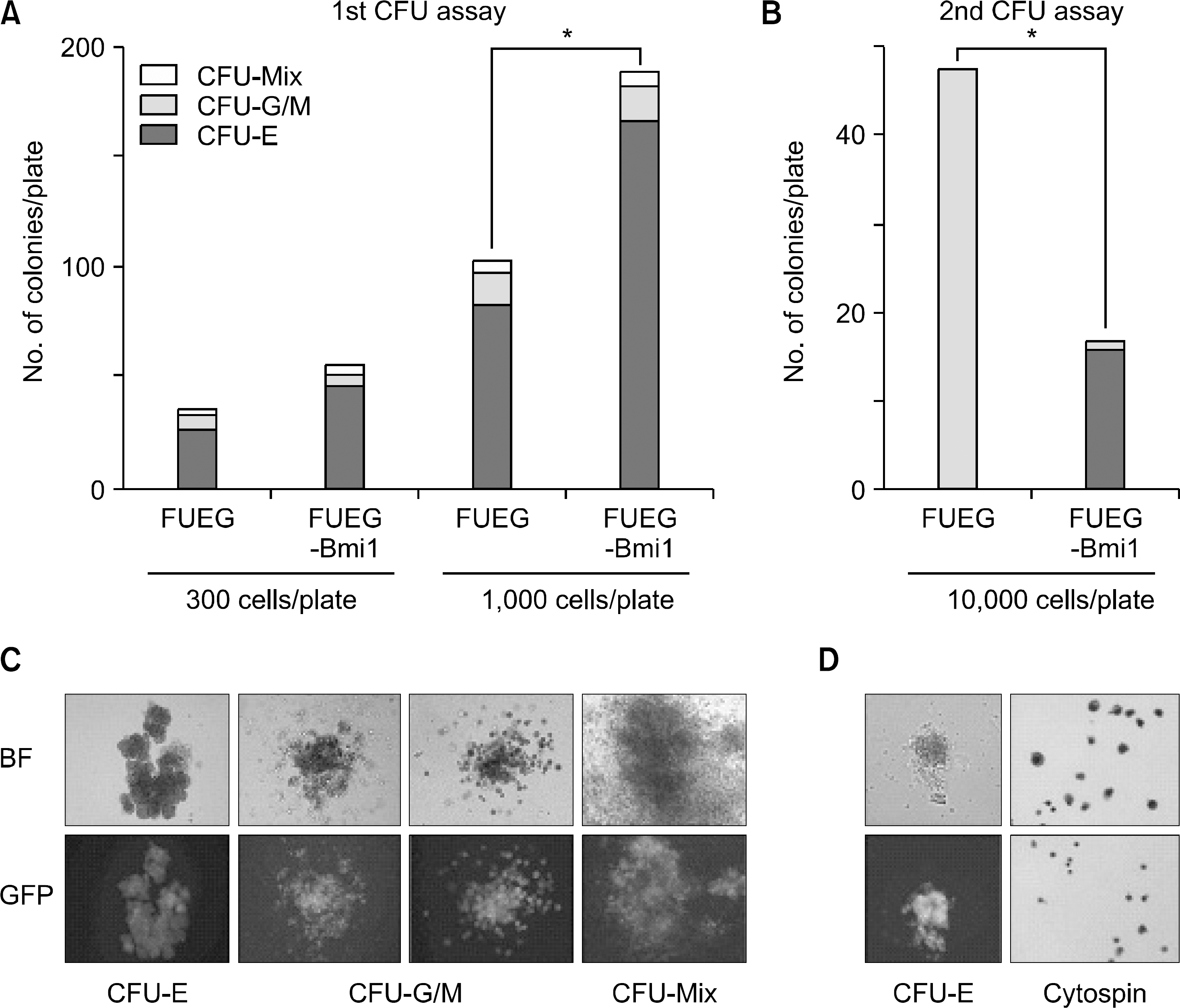
Fig. 3
The increase in number of erythroid colonies in the Bmi-1 CD34+ group is dependent on erythropoietin (Epo). Methylcellulose culture was established without Epo, with 1 unit/ml, and 6 units/ml of Epo, rspectively (A, B, C, respectively). KL (10ng/mL) was added in all cases. Significant differences in the number of erythroid colonies and in the total number of colonies were observed in the presence of 6 IU/ml of Epo (∗P<0.05).
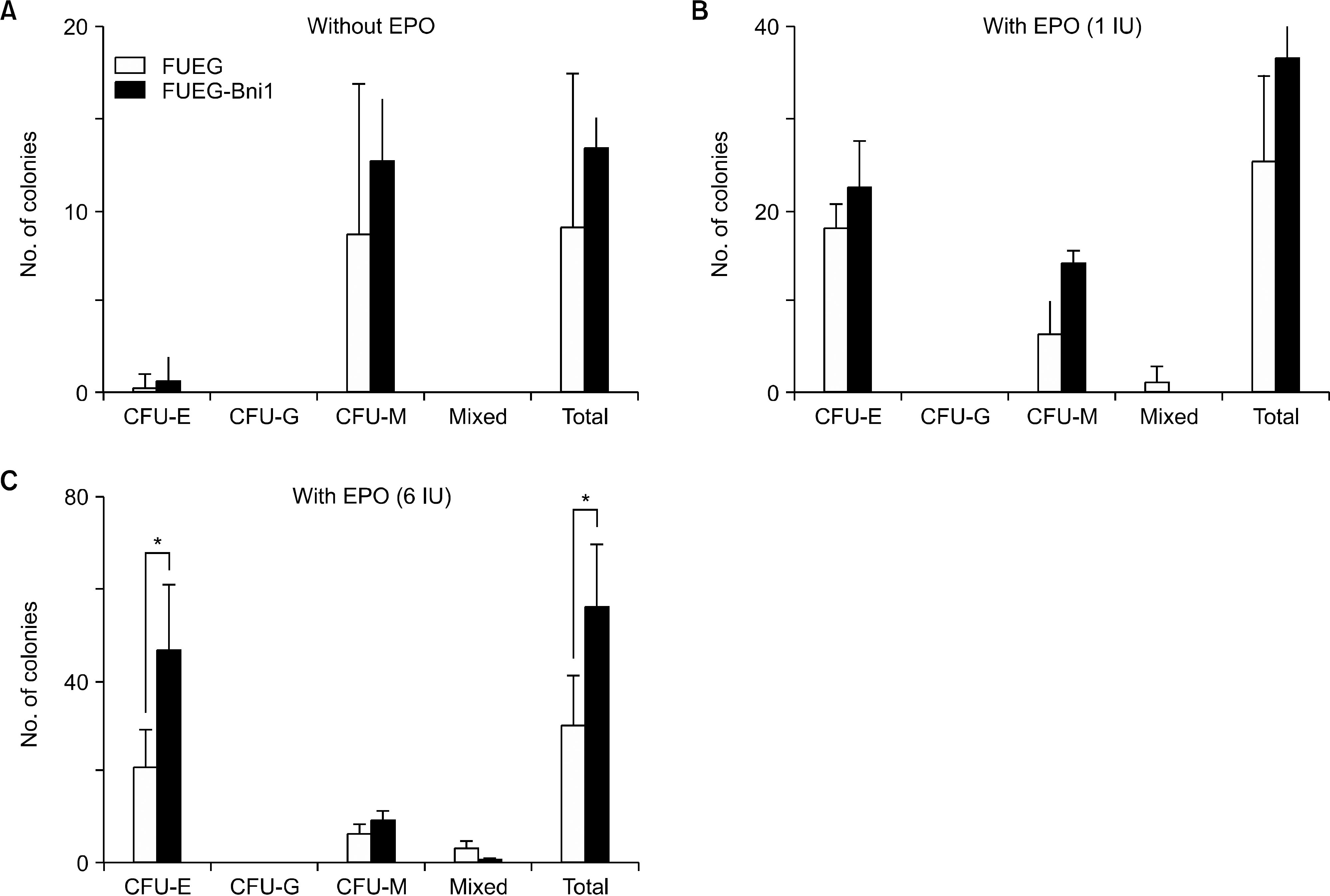
Fig. 4
Forced expression of Bmi-1 does not increase self-renewal of CB HSC. (A) Results of cobblestone area forming assay showed that there is no significant difference between non-transduced, FUEG and FUEG-Bmi-1-transduced groups (Error bar is not shown). (B, D) Number of non-adherent cells in co-cultures of CD34+ cells on OP-9 and MS-5 stromal cells was not different between the two groups (Error bar is not shown). (C, E) Methylcellulose cultures were established with non-adherent cells obtained from 5-week co-cultures on OP-9 and MS-5 stromal cells. A significant decrease in total number of 5 week colonies in FUEG-Bmi-1 group was observed.
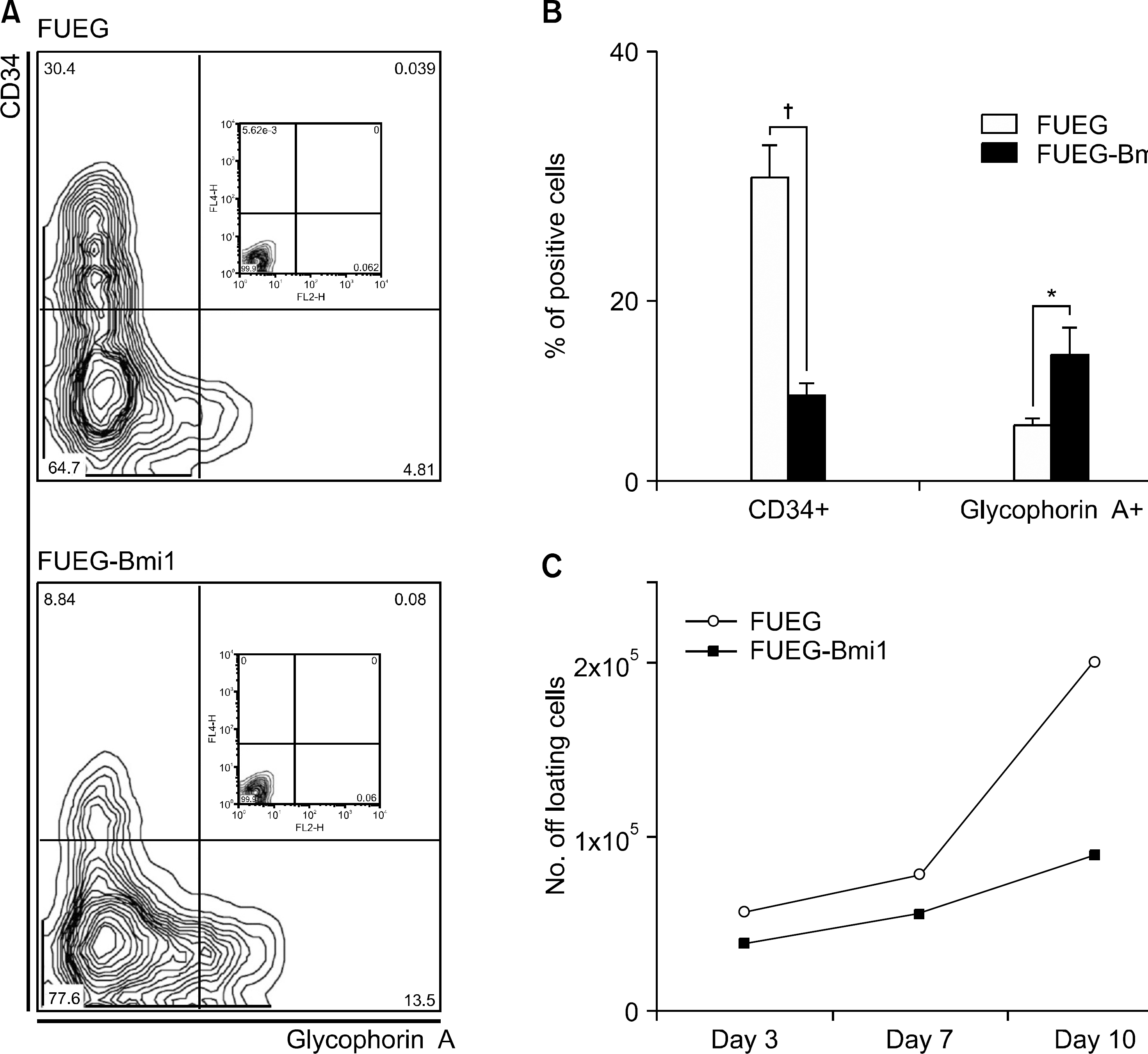
Fig. 5
Overexpression of Bmi-1 promotes erythroid differentiation of CB CD34+ cells. GFP+ CB CD34+ cells were cultured in serum-free medium supplemented with KL, Flt3 ligand, Tpo, and Epo. (A) Representative images of flow cytometric analysis using anti-CD34 and anti-Glycophorin A antibodies after 7 days of culture in serum-free condition. (B) Quantification of CD34+ and Glycophorin A+ cells in stroma free culture for 7 days showed a significant decrease of CD34+ cells and an increase of Glycophorin A+ cells. (C) Total number of the cells in stromal-free culture did not show significant difference (Error bar is not shown).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download