Abstract
Background:
Donor lymphocyte infusion (DLI) has been established as a salvage therapy for patients with relapsed leukemia after allogeneic hematopoietic stem cell transplantation (HSCT). However, its benefit can be limited by the development of graft-versus-host disease (GVHD) or marrow aplasia.
Methods:
We retrospectively analyzed the data from 39 patients that received DLI for relapsed leukemia after HLA-matched, related HSCT between 1995 and 2005 at Seoul National University Hospital.
Results:
The diagnoses were CML (n=8), AML (n=19) and ALL (n=12). Ten patients had received non-myeloablative HSCT (AML=9, ALL=1). Complete remission after DLI was achieved in 6 (75%) cases with CML, 5 cases (29%) with AML and 5 cases (41%) with ALL. The two-year progression-free survival was 60% in CML patients, but 8.1% in non-CML patients (P=0.01). In addition, better overall survival (OS) was shown in CML patients than in non-CML patients (2-year OS, 68% in CML; 10% in non-CML, P=0.01). The durable remission for more than three years after DLI was confirmed in five patients (one AML patient for 88 months, one ALL patient for 54 months, three CML patients for 38, 47 and 53 months). Acute GVHD (≥Grade II) developed in 14 patients (35.9%). Prolonged marrow aplasia (neutrophil count <500/μL, platelet count <20,000/μL) developed in fourpatients (10.3%).
REFERENCES
1). Mathe G., Schwarzenberg L. Bone marrow transplantation (1958-1978): conditioning and graft-versus-host disease, indications in aplasias and leukemias. Pathol Biol (Paris). 1979. 27:337–43.
2). Storb R., Santos GW. Application of bone marrow transplantation in leukaemia and aplastic anaemia. Clin Haematol. 1983. 12:721–37.

3). Horowitz MM., Gale RP., Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990. 75:555–62.

4). Weiden PL., Sullivan KM., Flournoy N., Storb R., Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981. 304:1529–33.
5). Transplant or chemotherapy in acute myelogenous leukaemia. International Bone Marrow Transplant Registry. Lancet. 1989. 1:1119–22.
6). Kumar L. Leukemia: management of relapse after allogeneic bone marrow transplantation. J Clin Oncol. 1994. 12:1710–7.

7). Mrsic M., Horowitz MM., Atkinson K, et al. Second HLA-identical sibling transplants for leukemia recurrence. Bone marrow transplant. 1992. 9:269–75.
8). Mortimer J., Blinder MA., Schulman S, et al. Relapse of acute leukemia after marrow transplantation: natural history and results of subsequent therapy. J Clin Oncol. 1989. 7:50–7.

9). Kolb HJ., Mittermuller J., Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990. 76:2462–5.

10). Kolb HJ., Schattenberg A., Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995. 86:2041–50.
11). Collins RH Jr., Shpilberg O., Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997. 15:433–44.

12). Zeiser R., Bertz H., Spyridonidis A., Houet L., Finke J. Donor lymphocyte infusions for multiple myeloma: clinical results and novel perspectives. Bone Marrow Transplant. 2004. 34:923–8.

13). Dey BR., McAfee S., Colby C, et al. Impact of prophylactic donor leukocyte infusions on mixed chimerism, graft-versus-host disease, and antitumor response in patients with advanced hematologic malignancies treated with nonmyeloablative conditioning and allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2003. 9:320–9.

14). Bethge WA., Hegenbart U., Stuart MJ, et al. Adoptive immunotherapy with donor lymphocyte infusions after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Blood. 2004. 103:790–5.

15). Lee S., Park S., Kim BK, et al. Donor leukocyte infusion as treatment for relapsed leukemia after allogeneic bone marrow transplantation: graft- versus-leukemia effect. Korean J Hematol. 1999. 34:252–62.
16). Glucksberg H., Storb R., Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974. 18:295–304.

17). Maris MB., Niederwieser D., Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003. 102:2021–30.

18). McSweeney PA., Niederwieser D., Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001. 97:3390–400.

19). Niederwieser D., Maris M., Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003. 101:1620–9.

20). Kolb HJ., Schattenberg A., Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995. 86:2041–50.
21). Weisser M., Tischer J., Schnittger S., Schoch C., Ledderose G., Kolb HJ. A comparison of donor lymphocyte infusions or imatinib mesylate for patients with chronic myelogenous leukemia who have relapsed after allogeneic stem cell transplantation. Haematologica. 2006. 91:663–6.
22). Savani BN., Montero A., Kurlander R., Childs R., Hensel N., Barrett AJ. Imatinib synergizes with donor lymphocyte infusions to achieve rapid molecular remission of CML relapsing after allogeneic stem cell transplantation. Bone Marrow Transplant. 2005. 36:1009–15.

23). Keil F., Haas OA., Fritsch G, et al. Donor leukocyte infusion for leukemic relapse after allogeneic marrow transplantation: lack of residual donor hematopoiesis predicts aplasia. Blood. 1997. 89:3113–7.

24). Mackinnon S., Papadopoulos EB., Carabasi MH, et al. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease. Blood. 1995. 86:1261–8.

Fig. 1
Progression free survival after donor lymphocyte infusion. (A) a. Chronic myelocytic leukemia. b. Acute lymphcytic leukemia. c. Acute myelocytic leukemia. (B) a. Chronic myelocytic leukemia. b. non-Chronic myelocytic leukemia.
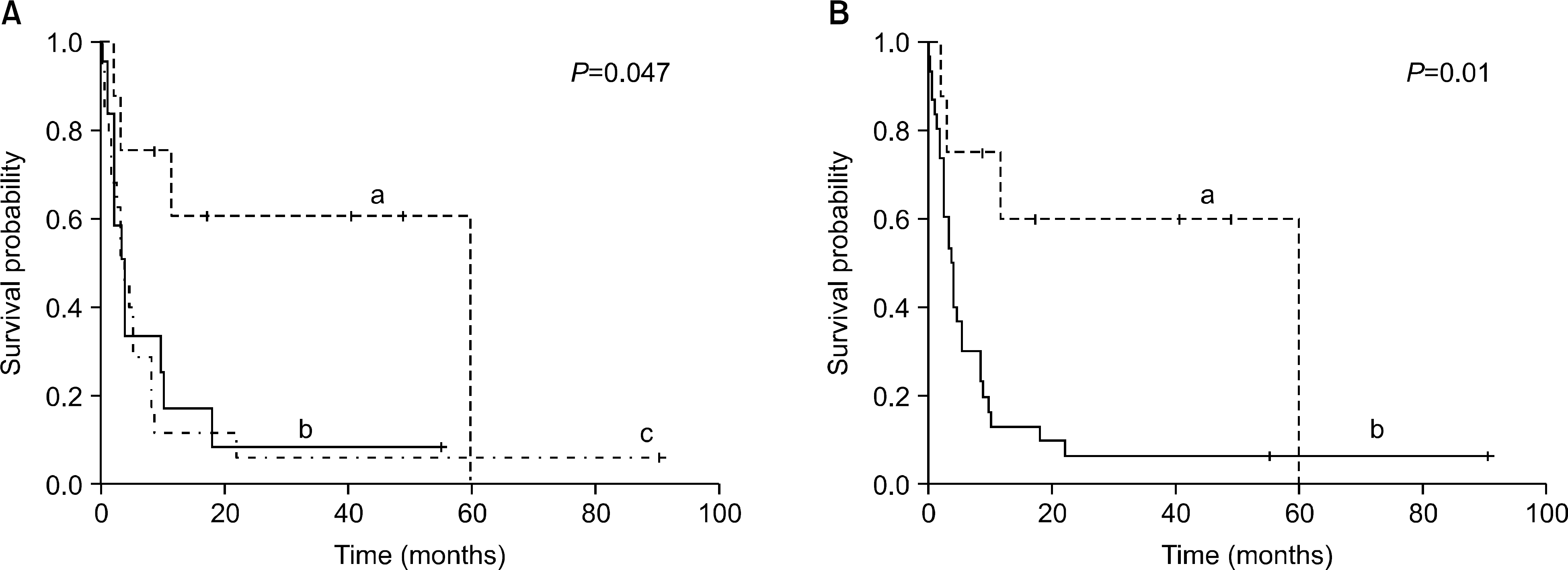
Fig. 2
Overall survival after donor lymphocyte infusion. (A) a. Chronic myelocytic leukemia. b. Acute lymphcytic leukemia. c. Acute myelocytic leukemia. (B) a. Chronic myelocytic leukemia. b. non-Chronic myelocytic leukemia.
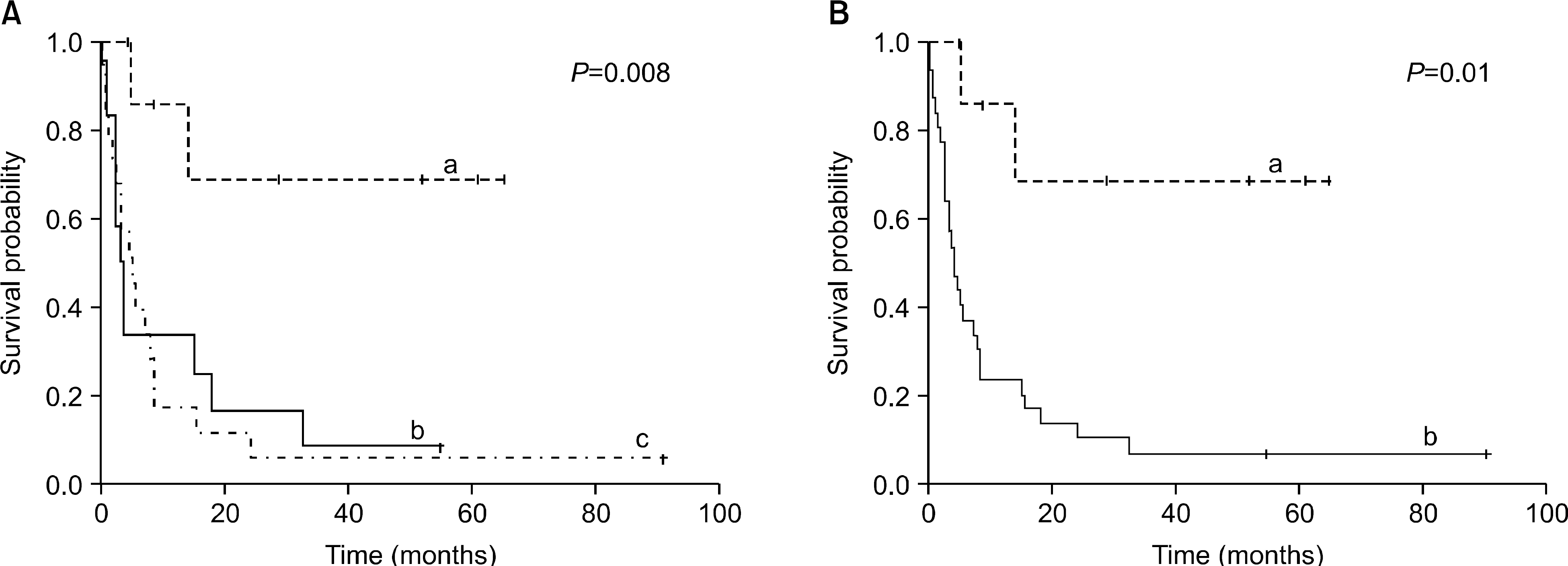
Table 1.
Baseline characteristics and donor lymphocyte infusion
Table 2.
Response after DLI and cell dose




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download