Abstract
Background:
Plasma coagulation factor XIII (FXIII) catalyzes the formation of covalent bounds between fibrin monomers, thus stabilizing the fibrin clot and increasing its resistance to fibrinolysis. Alteration of FXIII may contribute to bleeding, wound dehiscence and recurrent abortion. However, standard clotting tests cannot detect the FXIII deficiency. In this study, we evaluated a newly developed FXIII test kit (CoalinkTM, PeopleBio Inc., Seoul, Korea) in patients with various clinical conditions.
Methods:
We evaluated the linearity and precision of the new FXIII test kit and compared the results of the new kit and the Pefakit FXIII assay. The FXIII was tested in idiopathic thrombocytopenic purpura (ITP) (n=40) patients, chronic renal failure (CRF) (n=20) patients, liver cirrhosis (LC) (n=40) patients, EDTA-induced pseudothrombocytopenia (EDTAIP) (n=10) patients, and in normal healthy persons (n=50). In the normal healthy persons, we determined a complete blood count (CBC), Ed-highlight-the second (n=50) is redundant. prothrombin time (PT) measurement and activated partial prothrombin time (aPTT) measurement and evaluated the results using the two assays.
Results:
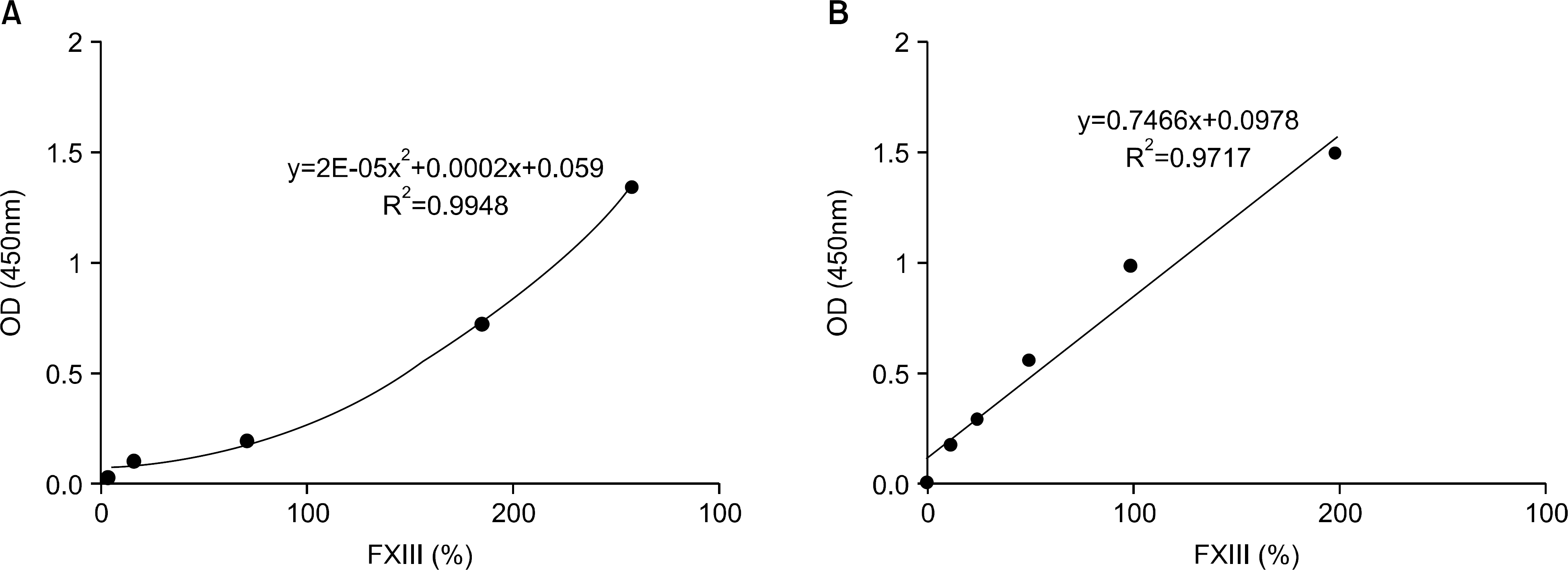
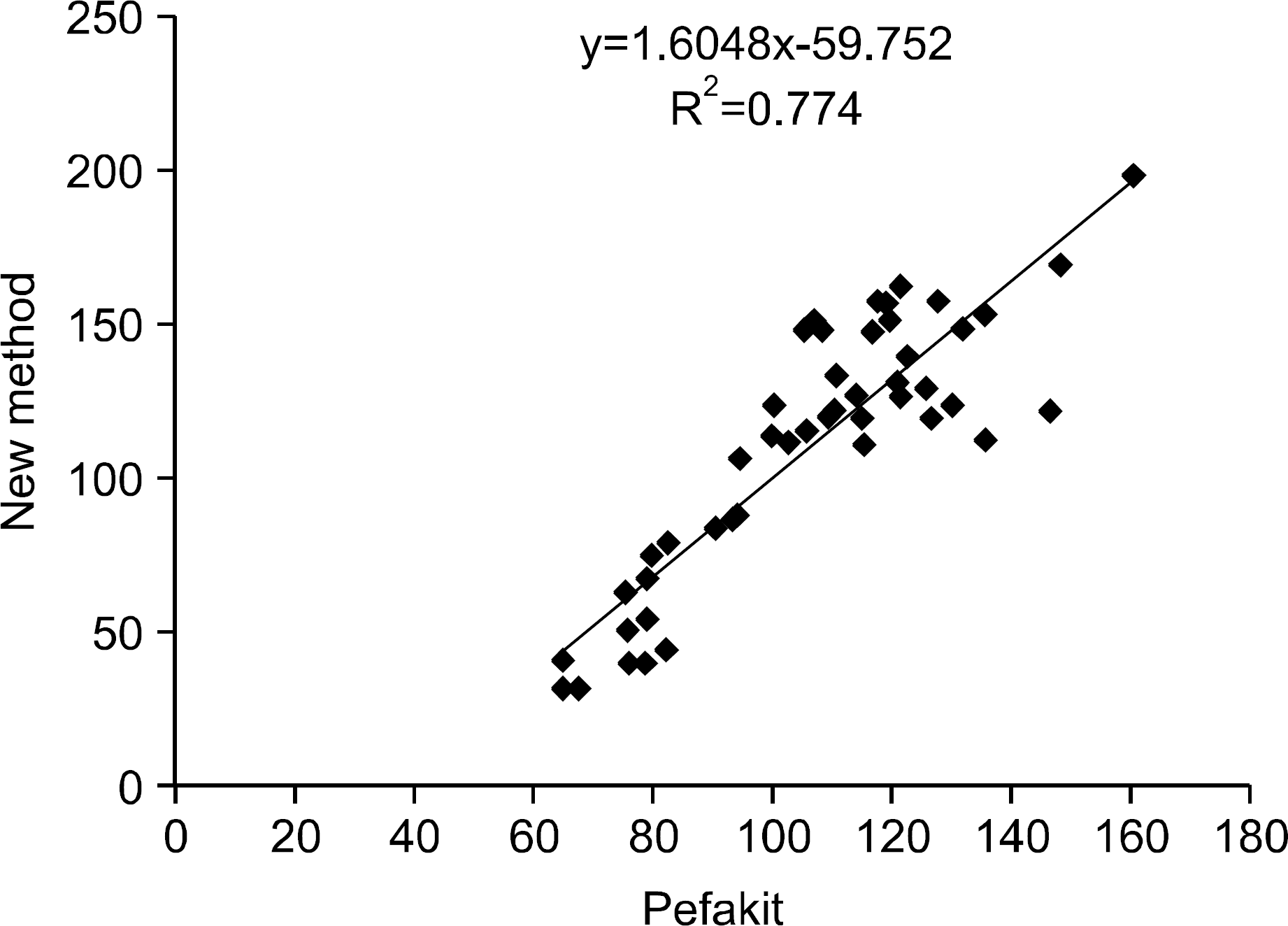
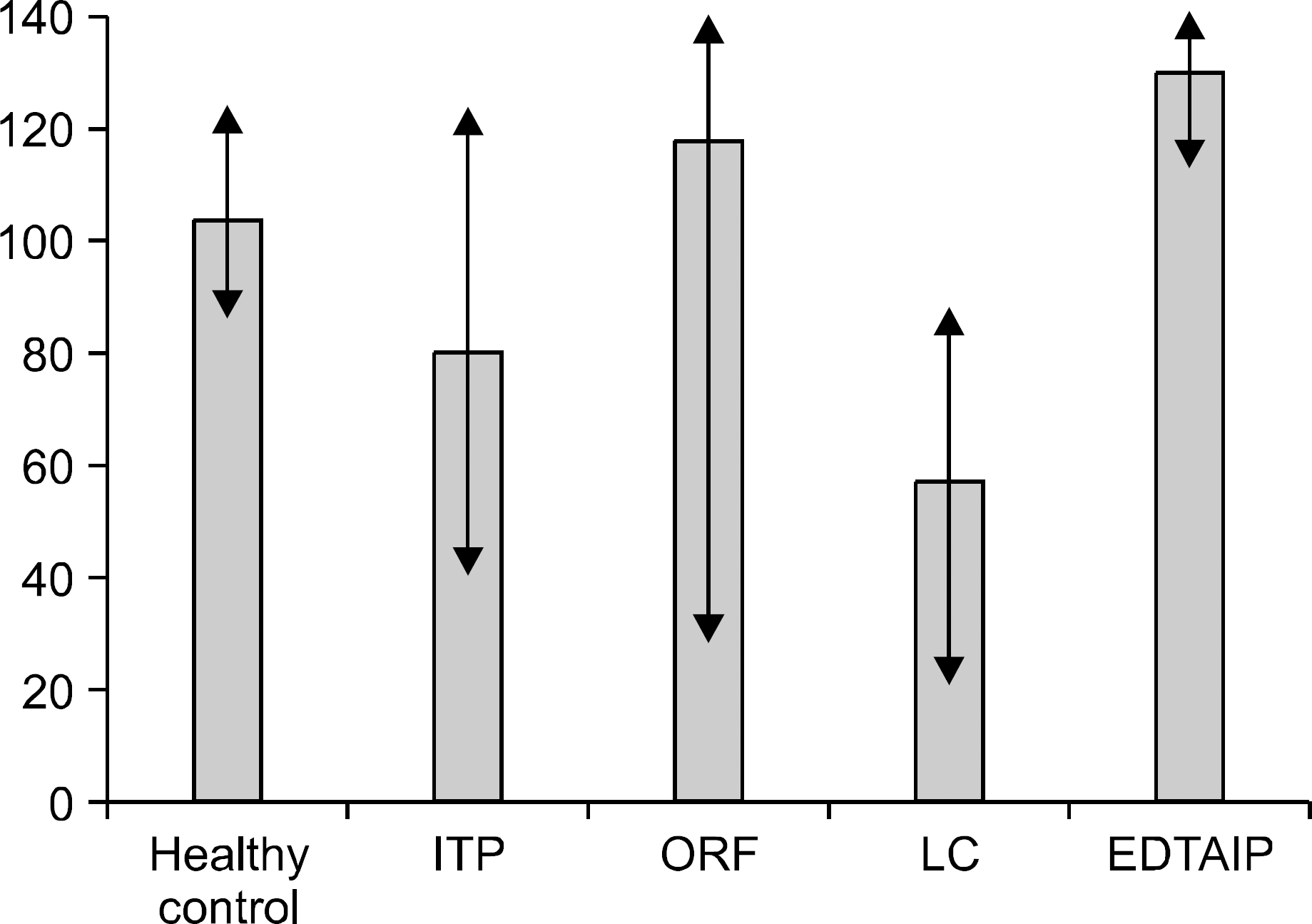
Serial dilution experiments with five samples provided good linearity (r2=0.9717). The intra-and inter assay precisions (CV) were 2.3∼8.6% and 3.9∼14.9%, respectively (n=20). There was a significant correlation between the use of the new kit and the Pefakit FXIII assay (r=0.8798, n=50). The FXIII activities of the normal healthy persons, ITP, CRF, LC and EDTAIP patients were 103.3±23.3%, 79.7± 41.0%, 117.9±82.3%, 56.9±23.7% and 130.0±29.0%, respectively and they were significantly decreased in the ITP and LC patients (P<0.05). The rates below 80% of the FXIII level were 67.5% in the ITP patients, 90.0% in the LC patients, 35.0% in the CRF patients and 0.0% in the EDTAIP patients. FXIII activities were closely related to platelet count (r=0.832, P<0.05) and negatively correlated with PT (r= ?0.389, P<0.05) and aPTT (r=?0.326, P<0.05).
REFERENCES
1). Muszbek L., Yee VC., Hevessy Z. Blood coagulation factor XIII: structure and function. Thromb Res. 1999. 94:271–305.
2). Hevessy Z., Haramura G., Boda Z., Udvardy M., Muszbek L. Promotion of the crosslinking of fibrin and alpha 2-antiplasmin by platelets. Thromb Hae-most. 1996. 75:161–7.
4). Tosetto A., Castaman G., Rodeghiero F. Acquired plasma factor XIII deficiencies. Haematologica. 1993. 78:5–10.
5). Seitz R., Leugner F., Katschinski M, et al. Ulcerative colitis and Crohn's disease: factor XIII, inflammation and haemostasis. Digestion. 1994. 55:361–7.

6). Seyfert UT., Hauck W., Helmling E., Albert FW. Factor XIII deficiency in adult polycystic kidney disease. Nephron. 1991. 58:365–6.

7). Franco RF., Pazin-Filho A., Tavella MH., Simoes MV., Marin-Neto JA., Zago MA. Factor XIII val34leu and the risk of myocardial infarction. Haematologica. 2000. 85:67–71.
8). Elbaz A., Poirier O., Canaple S., Chedru F., Cambien F., Amarenco P. The association between the Val34Leu polymorphism in the factor XIII gene and brain infarction. Blood. 2000. 95:586–91.

9). Catto AJ., Kohler HP., Coore J., Mansfield MW., Stickland MH., Grant PJ. Association of a common polymorphism in the factor XIII gene with venous thrombosis. Blood. 1999. 93:906–8.

10). Wells PS., Anderson JL., Scarvelis DK., Doucette SP., Gagnon F. Factor XIII Val34Leu variant is protective against venous thromboembolism: a HuGE review and meta-analysis. Am J Epidemiol. 2006. 164:101–9.

11). Gemmati D., Serino ML., Ongaro A, et al. A common mutation in the gene for coagulation factor XIII-A (VAL34Leu): a risk factor for primary intracerebral hemorrhage is protective against atherothrombotic diseases. Am J Hematol. 2001. 67:183–8.

12). Francis JL. The detection and measurement of factor XIII activity: a reiview. Med Lab Sci. 1980. 37:137–47.
13). Fickenscher K., Aab A., Stuber W. A photometric assay for blood coagulation factor XIII. Thromb Haemost. 1991. 65:535–40.

14). Mousli S., Wakid NW. Ammonia production during clot retraction and its use in assay of fibrinoligase. Clin Chem. 1977. 23:1739–43.

15). Lorand L., Campbell-Wilkes LK., Cooperstein L. A filter paper assay for transamidating enzymes using radioactive amine substrates. Anal Biochem. 1972. 50:623–31.

16). Lorand L., Lockridge OM., Campbell LK., Myhrman R., Bruner-Lorand J. Transamidating enzymes. II. A continuous fluorescent method suited for automating measurements of factor XIII in plasma. Anal Biochem. 1971. 44:221–31.
17). Lorand L., Parameswaran KN., Velasco PT., Hsu LK., Siefring GE Jr. New colored and fluorescent amine substrates for activated fibrin stabilizing factor (Factor XIIIa) and for transglutaminase. Anal Bio-chem. 1983. 131:419–25.

18). Lee KN., Birckbichler PJ., Patterson MK Jr. Colorimetric assay of blood coagulation factor XIII in plasma. Clin Chem. 1988. 34:906–10.

19). Slaughter TF., Achyuthan KE., Lai TS., Greenberg CS. A microtiter plate transglutaminase assay utilizing 5-(biotinamido)pentylamine as substrate. Anal Biochem. 1992. 205:166–71.

20). Ariens RA., Lai TS., Weisel JW., Greenberg CS., Grant PJ. Role of factor XIII in fibrin clot formation and effects of genetic polymorphisms. Blood. 2002. 100:743–54.
21). Van Bodegraven AA., Tuynman HA., Schoorl M., Kruishoop AM., Bartels PC. Fibrinolytic split products, fibrinolysis, and factor XIII activity in inflammatory bowel disease. Scand J Gastroenterol. 1995. 30:580–5.

22). Tacke F., Fiedler K., von Depka M, et al. Clinical and prognostic role of plasma coagulation factor XIII activity for bleeding disorders and 6-year survival in patients with chronic liver disease. Liver Int. 2006. 26:173–81.

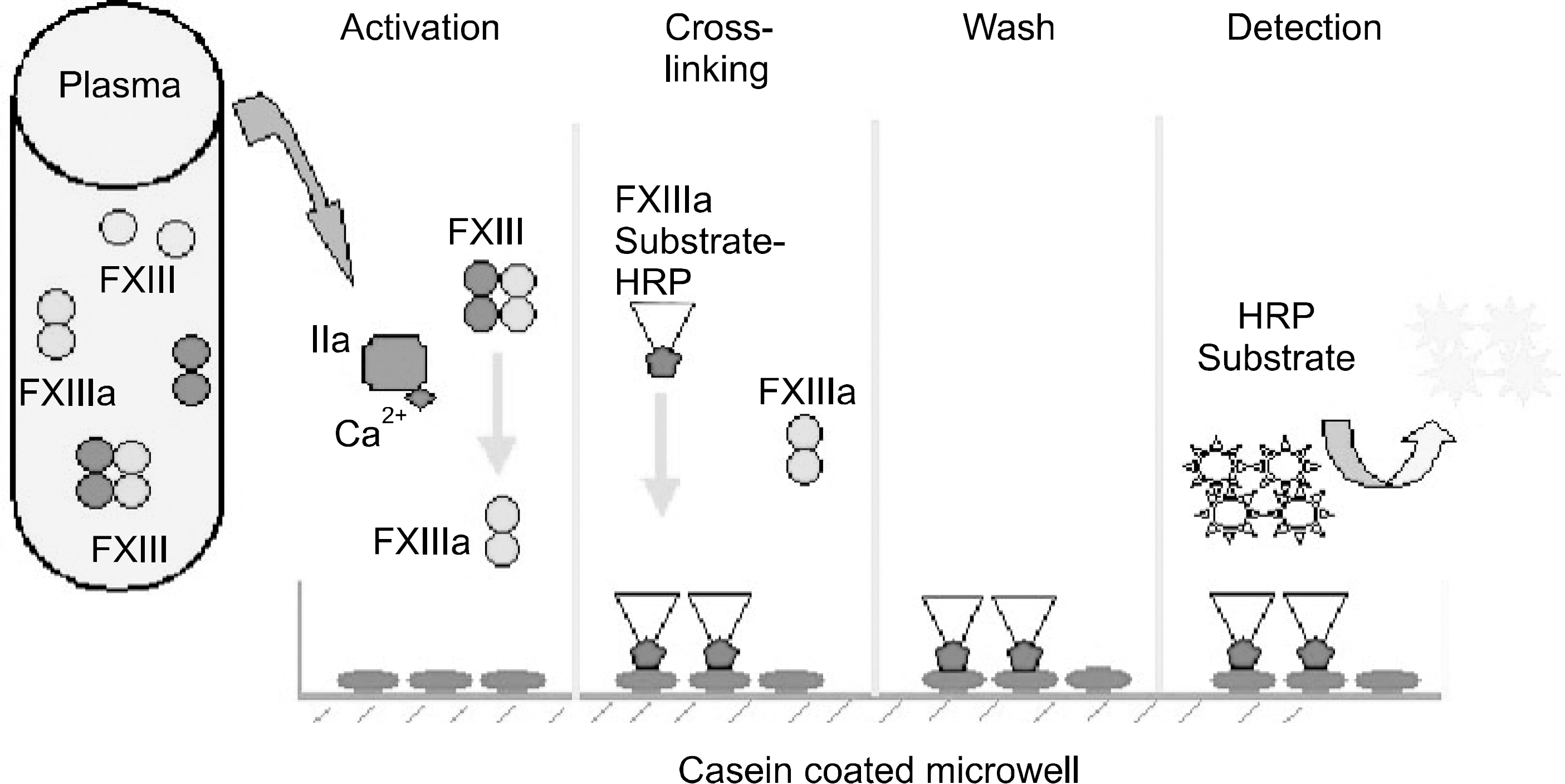
Fig. 1
Quantitative measurement of FXIII and FXIIIa activities in clinical sample by using the transglutaminase activity. The mechanism is based on the cross-linking the HRP conjugated FXIII fibrinogen complex to casein coated plate by thrombin activated FXIIIa, which is in the clinical sample. The crosslinked HRP is detected with HRP chromogenic substrate.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download