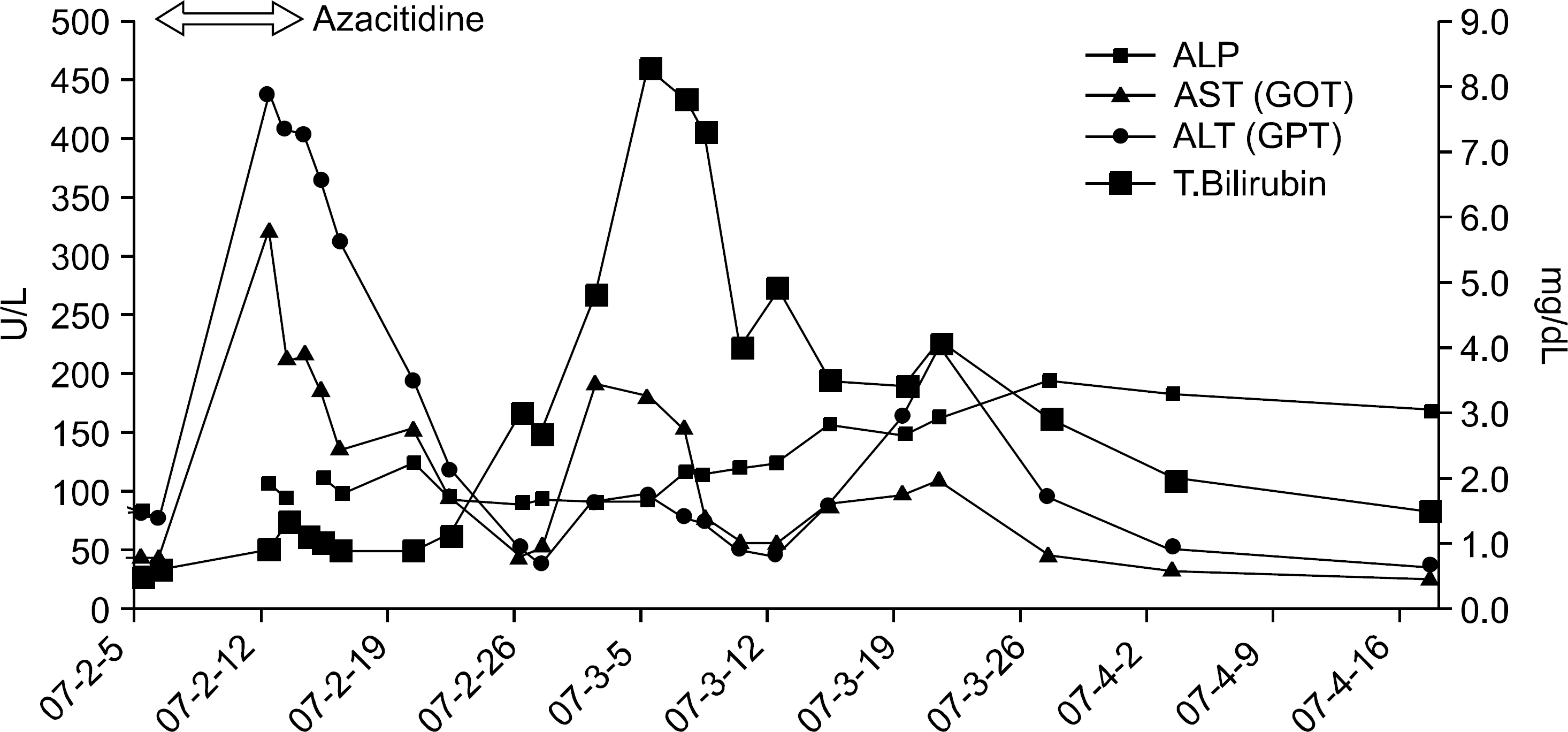
Abstract
Hematopoietic stem cell transplantation remains the only curative option for myelodysplastic syndrome (MDS), but the prevalence of the disease in elderly people limits broad application of the procedure, particularly in lower risk group. Azacitidine has been recently approved by the U.S. Food and Drug Administration for MDS regardless of subtype on French-American-British classification. Adverse effects of azacitidine include gastrointestinal, hematological and infusion-related reactions. Azacitidine induced hepatotoxicity has been reported mainly in patients with previous hepatobiliary disease, e.g., extensive tumor infiltration in liver, liver cirrhosis and cholelithiasis. We report here a case of azacitidine-induced hepatitis under no predisposition to hepatobiliary disease.
Go to : 
REFERENCES
1). Schiffer CA. Clinical issues in the management of patients with myelodysplasia. Hematology Am Soc Hematol Educ Program. 2006. 205–10.

2). Vardiman JW., Harris NL., Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002. 100:2292–302.

3). Silverman LR., Demakos EP., Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002. 20:2429–40.

4). Kaminskas E., Farrell AT., Wang YC., Sridhara R., Pazdur R. FDA drug approval summary: azacitidine (5-azacytidine, Vidaza) for injectable suspension. Oncologist. 2005. 10:176–82.

5). Pharmion. Vidaza Full Prescribing Information (Accessed April 10, 2007, at. http://www.vidaza.com/corporateweb/vidazaus/homeb.nsf/AttachmentsBy-Title/FullPrescribingInformation/FILE/FullPrescribisngInformationforVidaza.pdf).
6). Silverman LR., McKenzie DR., Peterson BL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006. 24:3895–903.

7). Von Hoff DD., Slavik M., Muggia FM. 5-Azacytidine. A new anticancer drug with effectiveness in acute myelogenous leukemia. Ann Intern Med. 1976. 85:237–45.
8). U.S. Food and Drug Administration. Vidaza's Approved Label. (Accessed May 15, 2007, at. http://www.fda.gov/cder/foi/label/2004/050794lbl.pdf.
9). Greenberg P., Cox C., LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997. 89:2079–88.

10). Ingram W., Lim ZY., Mufti GJ. Allogeneic transplantation for myelodysplastic syndrome (MDS). Blood Rev. 2007. 21:61–71.

11). Estey E., de Lima M., Tibes R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS). Blood. 2007. 109:1395–400.

12). Jädersten M., Montgomery SM., Dybedal I., Porwit-MacDonald A., Hellström-Lindberg E. Long-term outcome of treatment of anemia in MDS with erythropoietin and G-CSF. Blood. 2005. 106:803–11.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download