Abstract
Background:
Despite of aggressive treatments, the long-term survival rate is about 30% in stage 4 neuroblastoma (NBL). Recently, dendritic cell (DC)-based immunotherapy is emerging as a promising tool in cancer treatment. But it is very difficult to get sufficient amount of autologous tumor as the source of tumor antigen in DC-based immunotherapy. The purpose of this study was to test whether DCs loaded with allogeneic NBL tumor antigens can prime effective antitumor immune responses.
Methods:
Peripheral blood mononuclear cells (PBMCs) were differentiated into immature DCs in the presence of GM-CSF and IL-4. As the source of tumor antigens, human neuroblastoma cell lines, SK-N-MC, SK-N-SH, and IMR-32, were used after induction of apoptosis by UV irradiation. The immature DCs were loaded with apoptotic tumor cells, and then cultured with PBMCs for priming the tumor antigen-specific T lymphocytes. The tumor-specific cytotoxicity of T lymphocytes against NBL cells was analysed after coculture.
Results:
Incubation of DCs with apoptotic tumor cells effectively loaded DCs with tumor antigens and induced maturation of DCs. The tumor antigen-challenged T lymphocytes effectively killed the NBL cells, which were used as tumor antigens. Furthermore, the T lymphocytes showed a broad ranged cytotoxicity to all of the NBL cell lines, which were not challenged as tumor antigens.
REFERENCES
1). Cheung NK., Kushner BH., LaQuaglia M, et al. N7: a novel multi-modality therapy of high risk neuroblastoma (NB) in children diagnosed over 1 year of age. Med Pediatr Oncol. 2001. 36:227–30.

2). Toren A., Nagler A., Rozenfeld-Granot G, et al. Amplification of immunological functions by subcutaneous injection of intermediate-high dose interleukin-2 for 2 years after autologous stem cell transplantation in children with stage IV neuroblastoma. Transplantation. 2000. 70:1100–4.

3). Abbas AK., Lichtman AH., Pober JS. Immunity to tumors. In: Cellular and molecular immunology. 3rd ed.Philadelphia: WB Saunders Co;1997. p. 382–405.
4). Banchereau J., Steinman RM. Dendritic cells and the control of immunity. Nature. 1998. 392:245–52.

5). Brossart P., Wirths S., Brugger W., Kanz L. Dendritic cells in cancer vaccines. Exp Hematol. 2001. 29:1247–55.

6). Bowman LC., Grossmann M., Rill D, et al. Interleukin-2 gene-modified allogeneic tumor cells for treatment of relapsed neuroblastoma. Hum Gene Ther. 1998. 9:1303–11.

7). Berard F., Blanco P., Davoust J, et al. Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J Exp Med. 2002. 192:1535–44.

8). Märten A., Greten T., Ziske C, et al. Generation of activated and antigen-specific T cells with cytotoxic activity after coculture with dendritic cells. Cancer Immunol Immunother. 2002. 51:25–32.
9). Lee GK. Characterization of new monoclonal antibodies recognizing subsets of human dendritic cells. The 43rd Biannual Meeting of the Korean Association of Immunologists, Abstract (SIII-2), pp47-48. 2001.
10). Kim JI., Lee HR., Kim EY., Kim SW., Lee GK., Song HG. Characterization of a new monoclonal antibody, CH6, against a human cell membrane molecule. The 42nd Biannual Meeting of the Korean Association of Immunologists, Abstract (P-06), p68. 2001.
11). Thurner B., Haendle I., Röder C, et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999. 190:1669–78.

12). Brossart P., Wirths S., Stuhler G., Reichardt VL., Kanz L., Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000. 96:3102–8.

13). Yu JS., Wheeler CJ., Zeltzer PM, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001. 61:842–7.
14). Nabel GJ., Nabel EG., Yang ZY, et al. Direct gene transfer with DNA-liposome complexes in melanoma: expression, biologic activity, and lack of toxicity in humans. Proc Natl Acad Sci U S A. 1993. 90:11307–11.

16). Pardoll D., Nabel GJ. Cancer immunotherapy. Friedmann T, editor. The development of human gene therapy. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press;1999. p. 427–57.
17). Zhao XJ., Cheung NK. GD2 oligosaccharide: target for cytotoxic T lymphocytes. J Exp Med. 1995. 182:67–74.

19). Foreman NK., Rill DR., Coustan-smith E., Douglass EC., Brenner MK. Mechanisms of selective killing of neuroblastoma cells by natural killer cells and lymphokine activated killer cells. Potential for residual disease eradication. Br J Cancer. 1993. 67:933–8.

20). Cheung NK., Medof ME., Munn D. Immunotherapy with GD2 specific monoclonal antibodies. Prog Clin Biol Res. 1988. 271:619–32.
21). Haight AE., Bowman LC., Ng CY., Vanin EF., Davi-doff AM. Humoral response to vaccination with interleukin-2-expressing allogeneic neuroblastoma cells after primary therapy. Med Pediatr Oncol. 2000. 35:712–5.

22). Eaton JD., Perry MJ., Nicholson S, et al. Allogeneic whole-cell vaccine: a phase I/II study in men with hormone-refractory prostate cancer. BJU Int. 2002. 89:19–26.

23). Melcher A., Todryk S., Bateman A., Chong H., Lemoi-ne NR., Vile RG. Adoptive transfer of immature dendritic cells with autologous or allogeneic tumor cells generates systemic antitumor immunity. Cancer Res. 1999. 59:2802–5.
Fig. 1
Scheme of the experimental design for stimulation of T lymphocytes by tumor antigen-loaded dendritic cells.
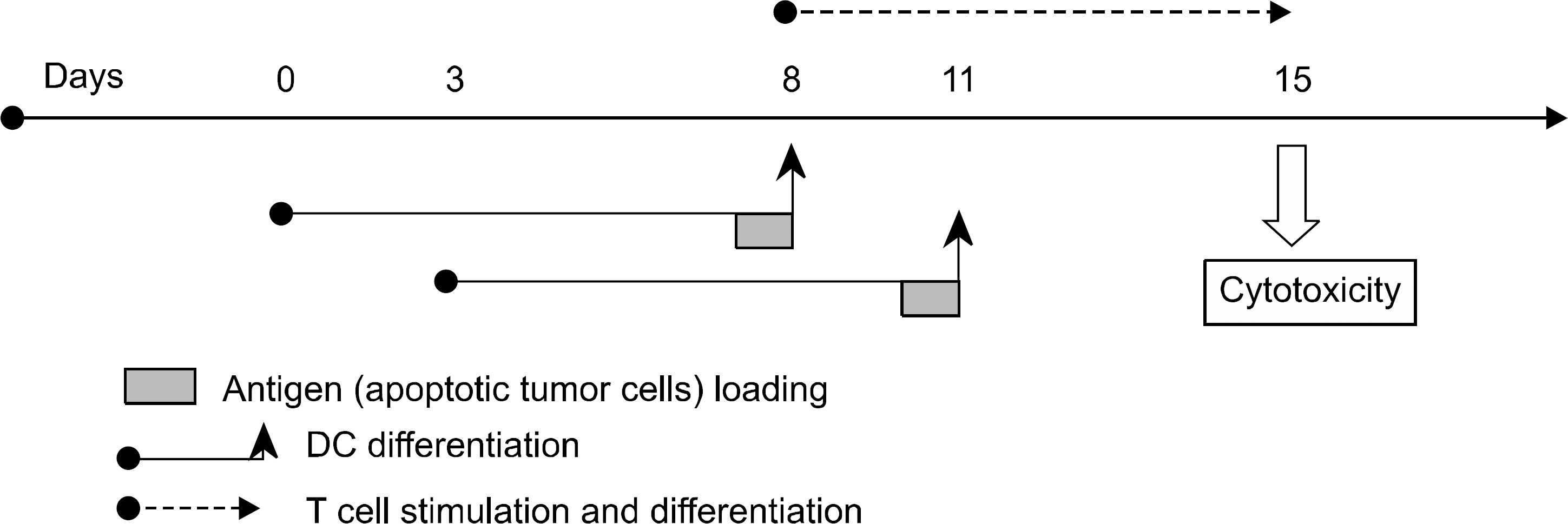
Fig. 2
Photomicrograph of peripheral blood monocyte- derived immature (left, ×400) and mature human dendritic cells (right, ×1,000), Papanicolaou staining.
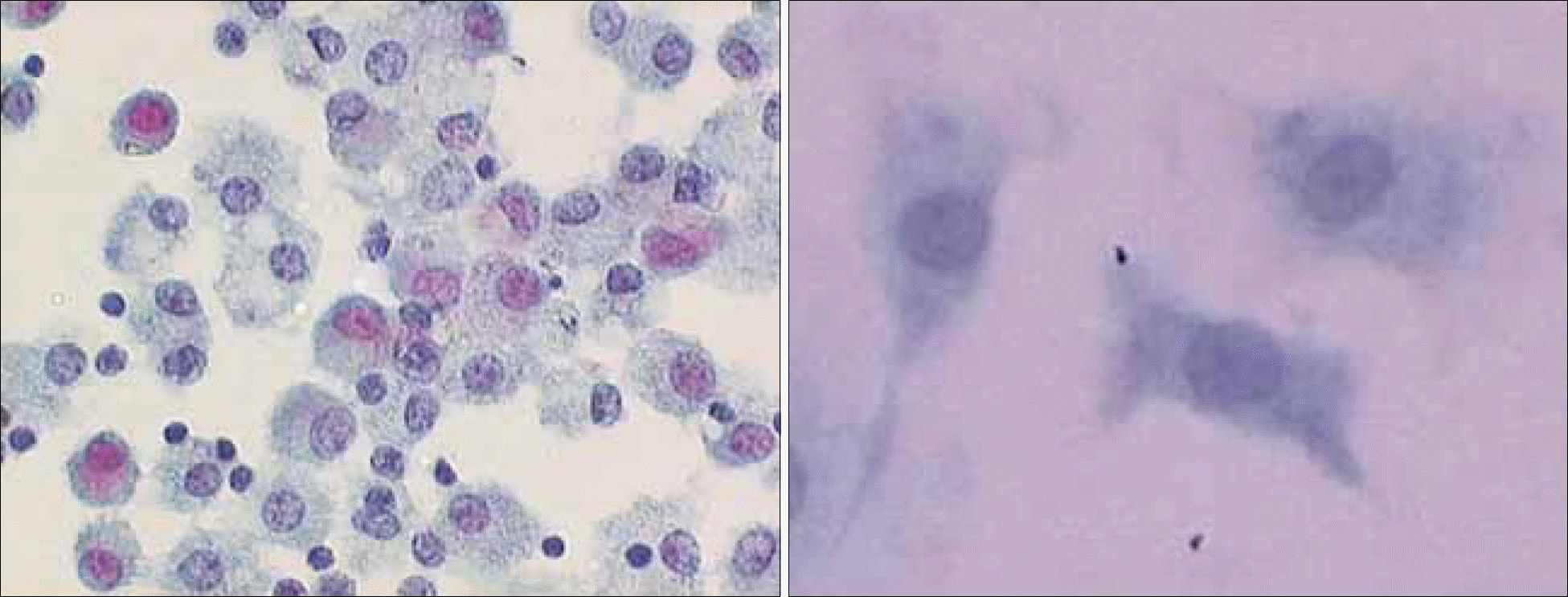
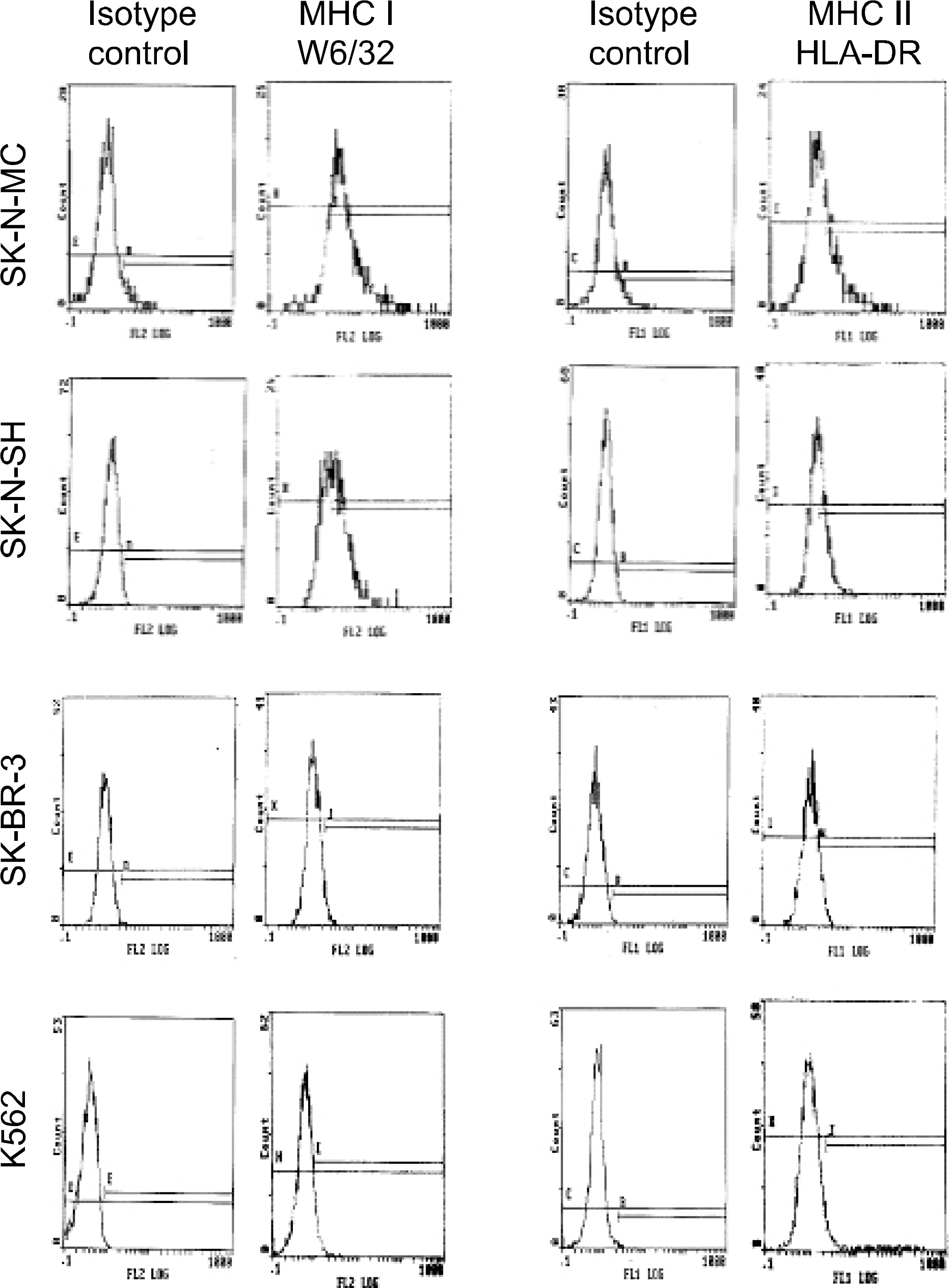
Fig. 3
Phenotypic analysis of peripheral blood monocyte-derived dendritic cells (PB-DC) by two color flow cytometry analysis using MoAb combinations of CH6, a new monoclonal antibody against human dendritic cells, and other markers of human dendritic cells.
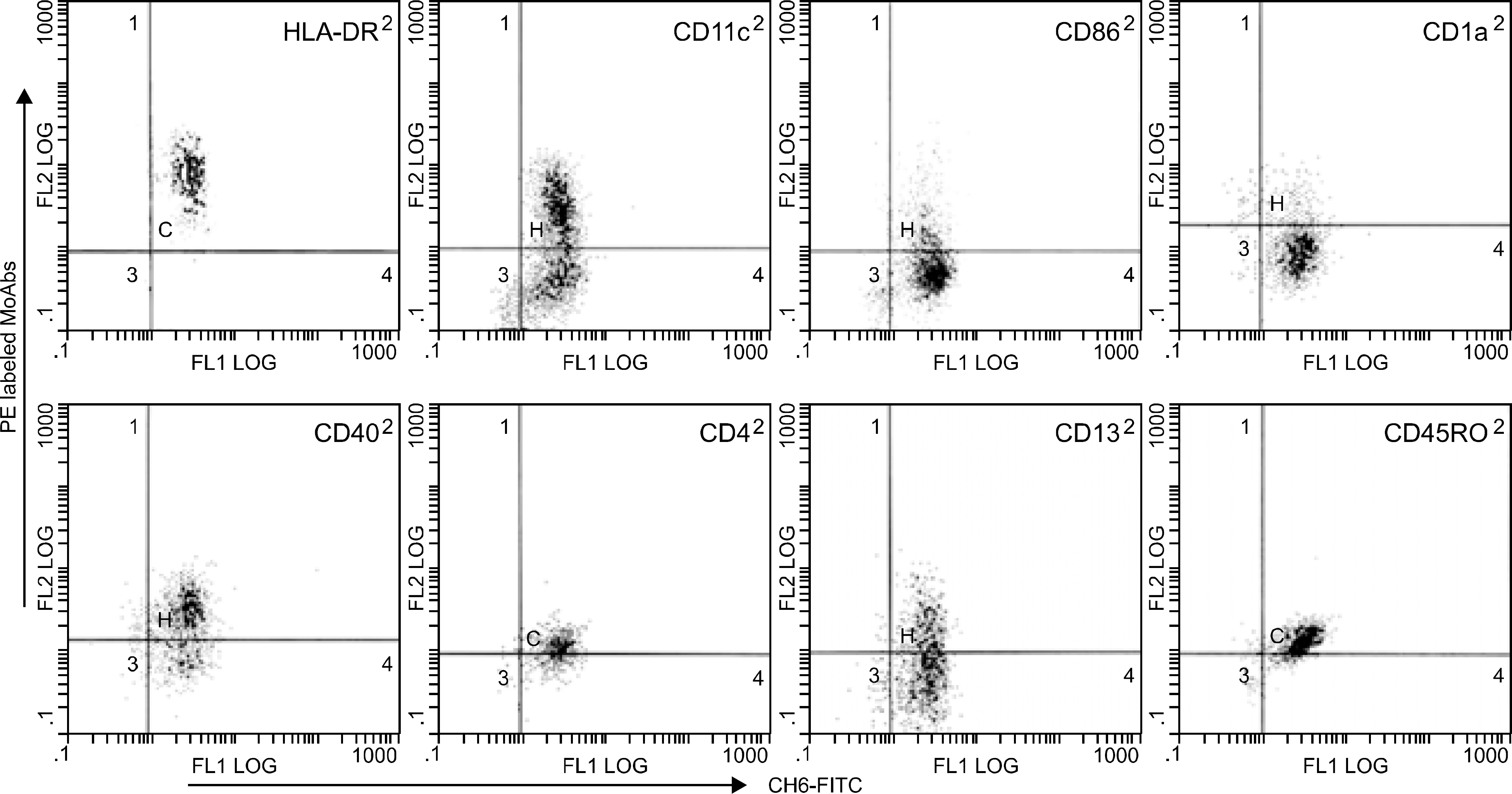
Fig. 4
UV-induced apoptosis of tumor cells (SK-N-MC). Apoptosis of the tumor cells was induced by UV irradiation for 2 hours at room temperature. Apoptosis and cell death were analysed by staining the cells with annexin V.
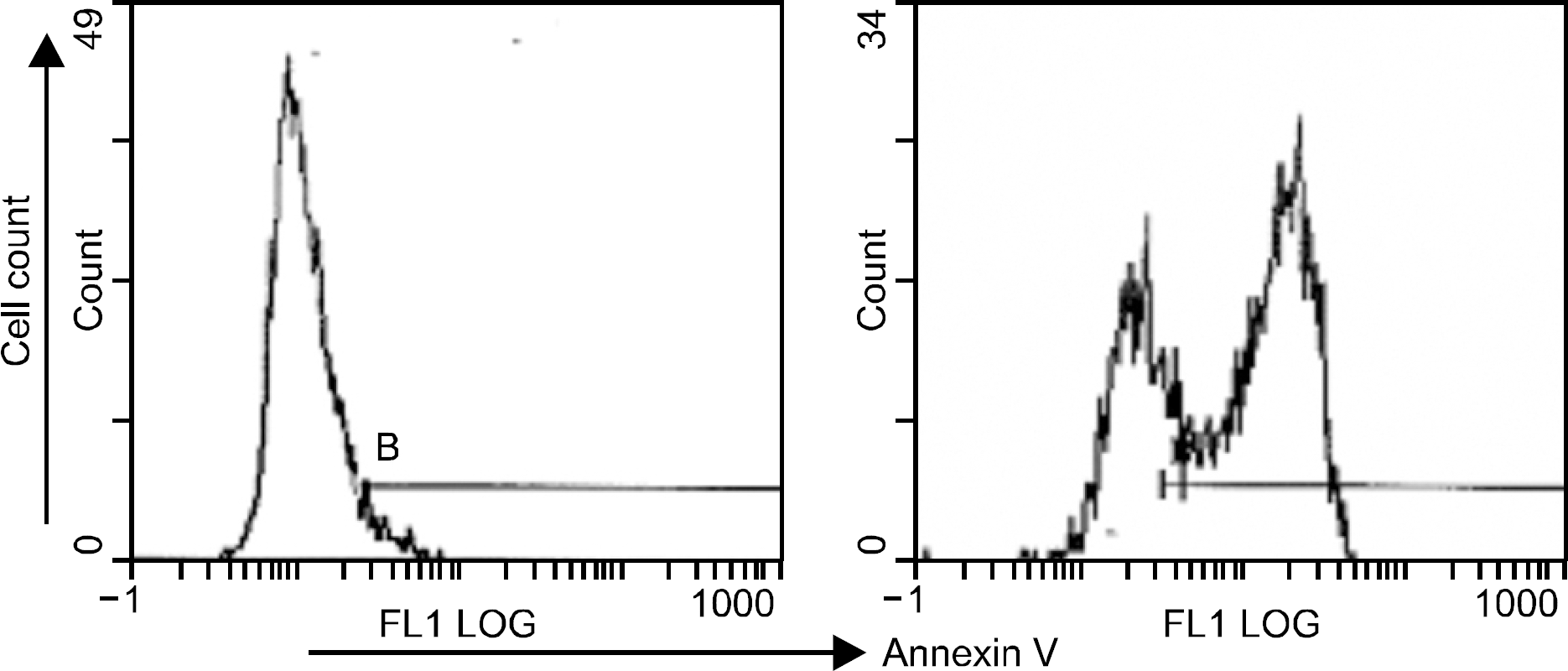
Fig. 5
Immunophenotypic changes of peripheral blood monocyte-derived dendrtitic cells before and after tumor antigen loading with apoptotic tumor cells (SK-N-SH).
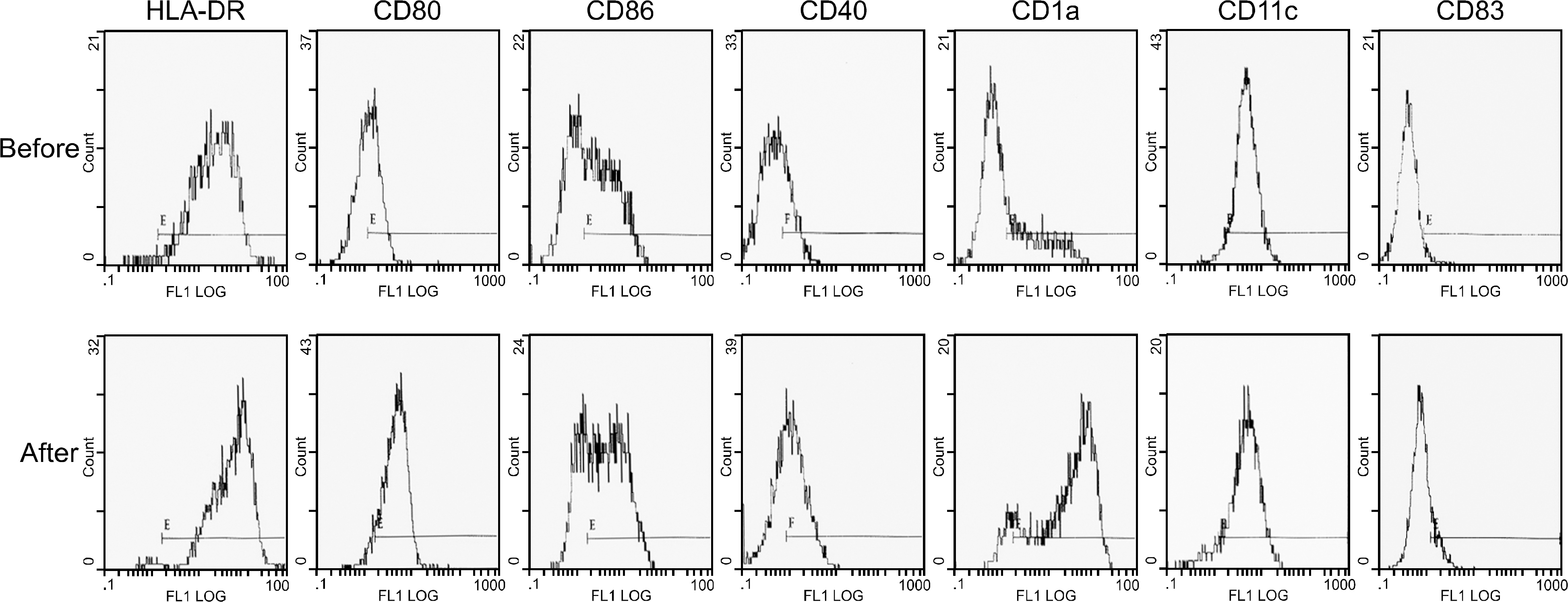
Fig. 6
Tumor-specific cytotoxicity by tumor antigen- challenged T lymphocytes. T lymphocytes were stimulated by tumor (SK-N-MC) antigen-loaded dendritic cells, and analysed for the tumor-specific cytotoxicity against three NBL cell lines; SK-N-MC (●—●), SK-N-SH (■—■), and IMR-32 (▲—▲), and two control cell lines; SK-BR-3 (△—△) and K-562 (○—○) at various effecto r:target (E:T) ratios.
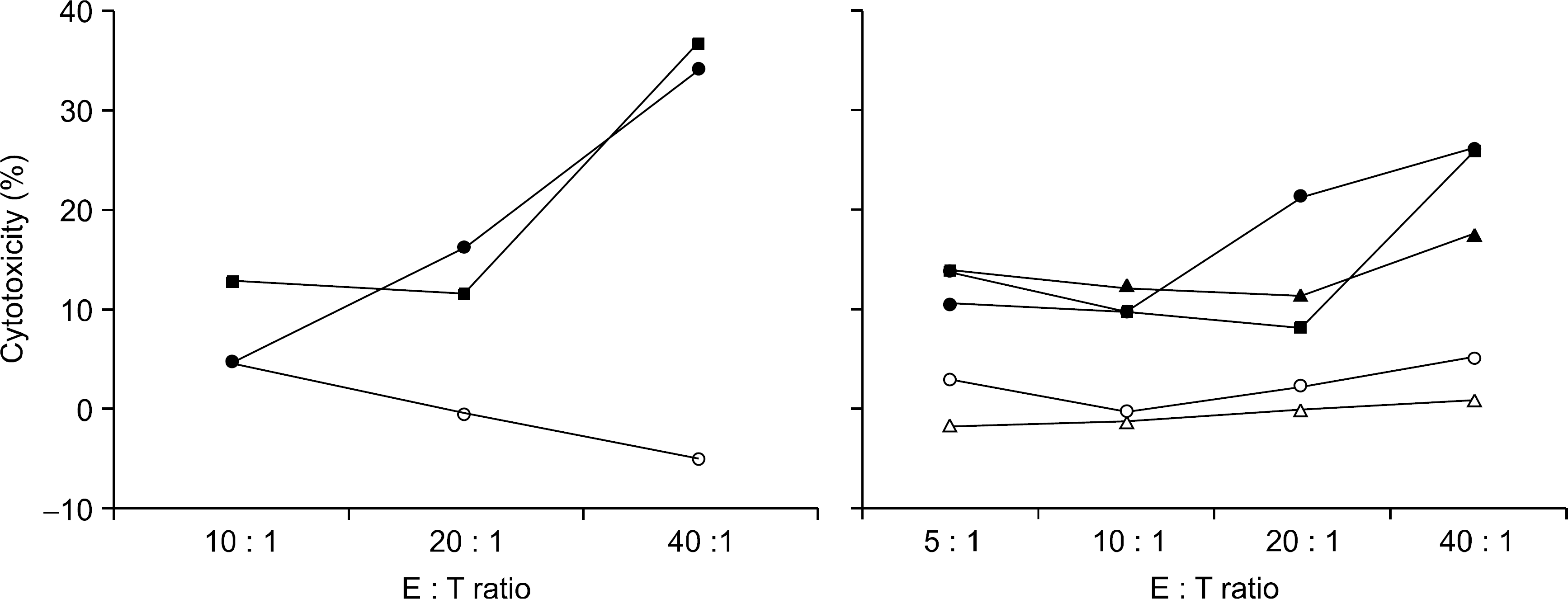
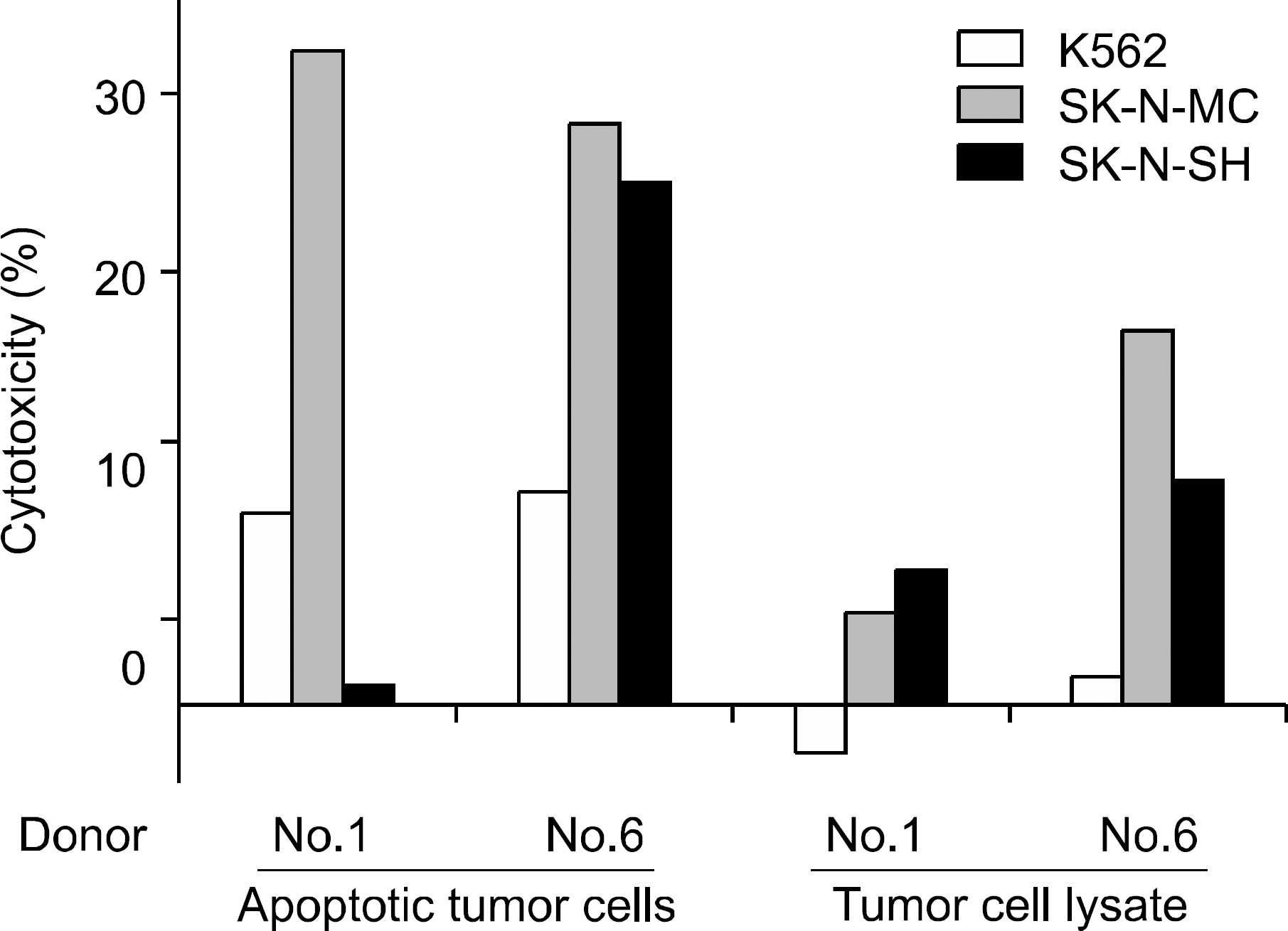
Fig. 7
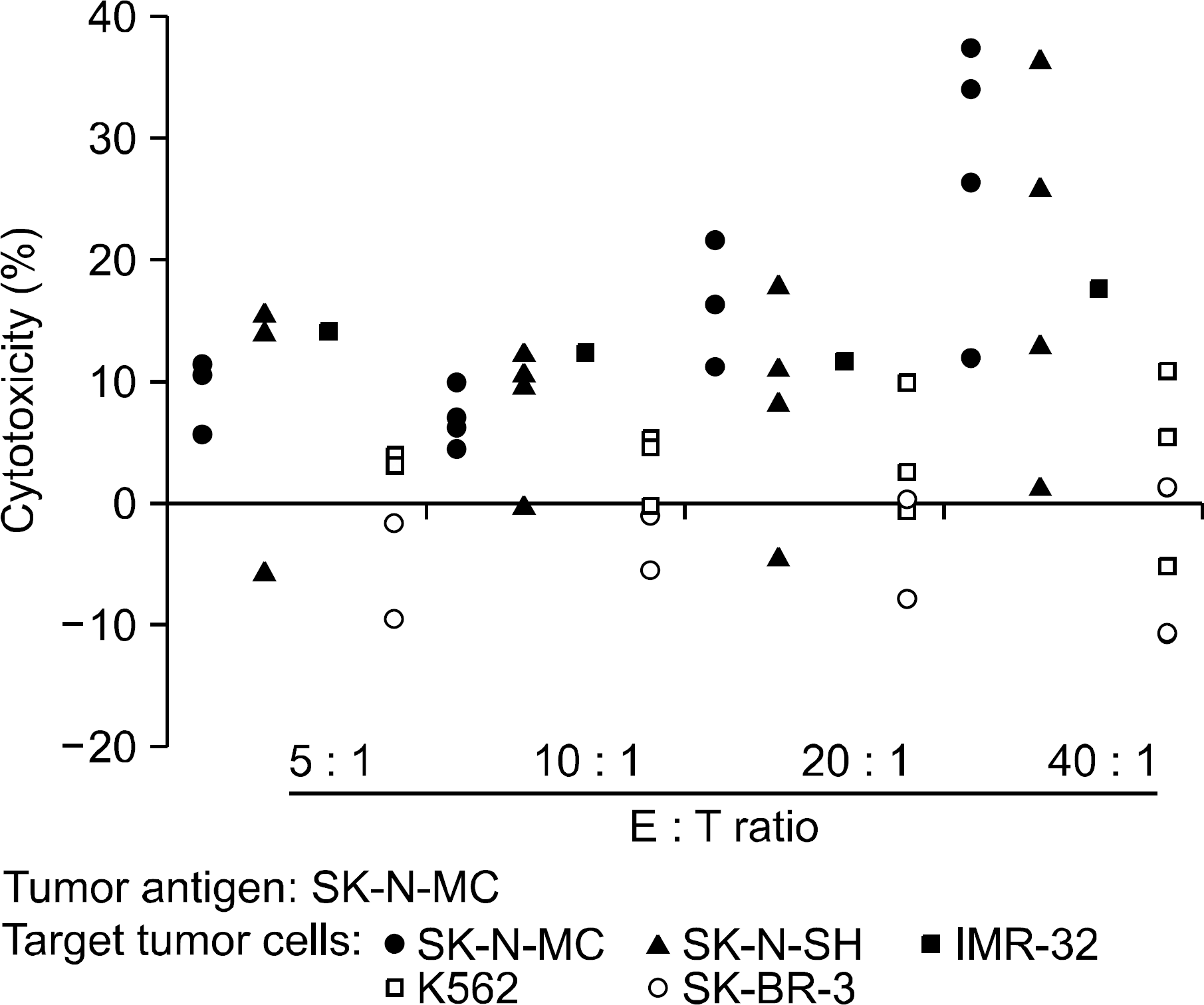
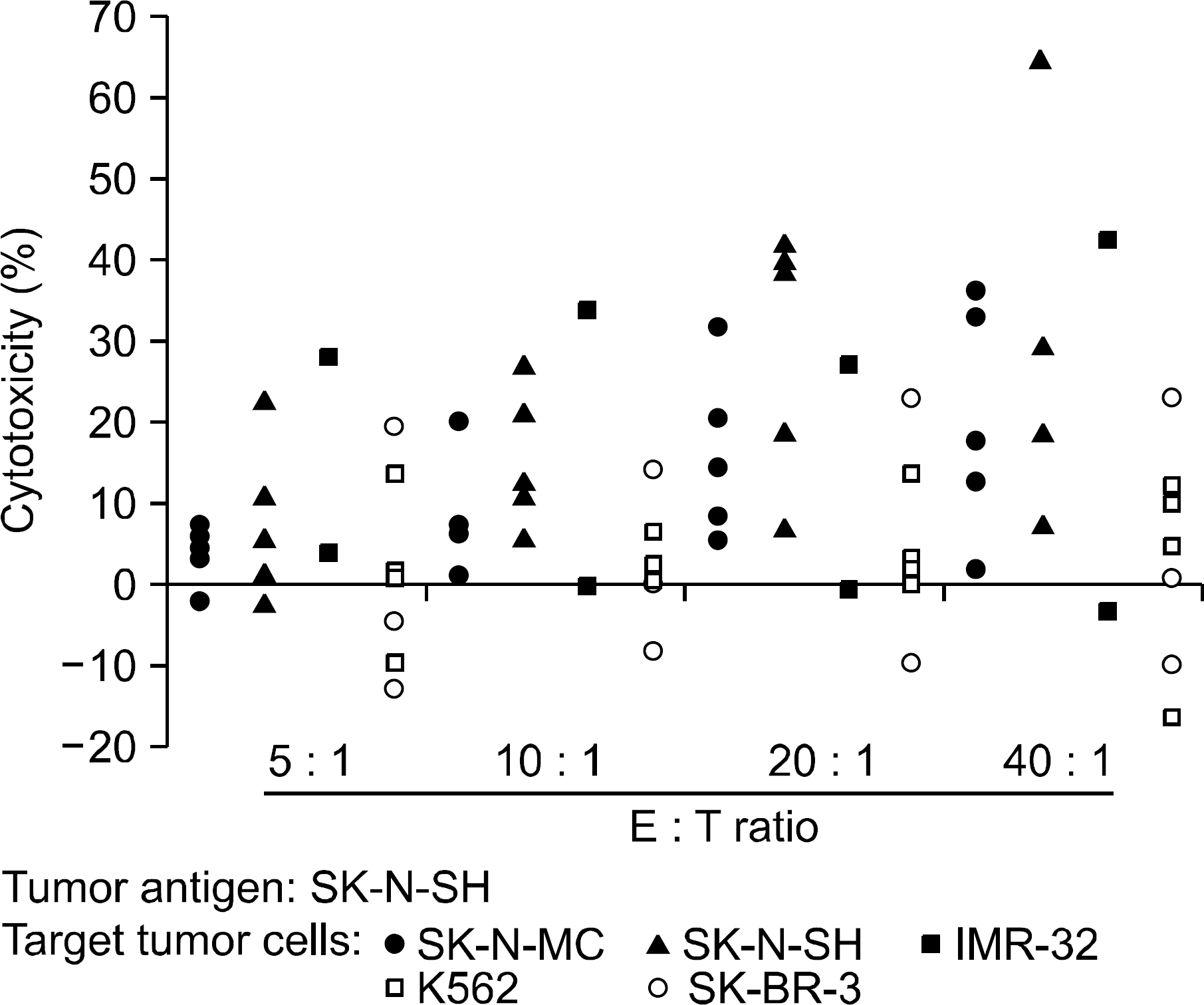
Tumor-directed cytotoxicity of T lymphocytes against various tumor cell lines at various effector:target (E:T) ratios. T lymphocytes from different donors were stimulated by DCs loaded with apoptotic SK-N-MC cells, and tumor-specific cytotoxic effector function was measured.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download