Abstract
Background:
The molecular mechanisms that are responsible for the initiation and progression of diffuse large B-cell lymphoma are largely unknown. p16 is an inhibitor of cyclin-dependent kinase and its inactivation by methylation has been reported as a major tumorigenic mechanism in malignant tumors. The aim of this study was to analyze the correlation between the clinical data and the p16 protein expression in diffuse large B-cell lymphomas.
Methods:
Tumor samples were obtained from 62 patients who were suffering with diffuse large B-cell lymphoma. To investigate the role of p16 in the pathogenesis and progression of diffuse large B-cell lymphoma, 62 cases of diffuse large B-cell lymphoma were examined for their expression of p16 via performing immunohistochemistry. The correlation of the p16 expression with the various clinicopathologic findings was also analyzed.
Results:
p16 was expressed in all the cases of reactive lymphoid hyperplasia (100%) and in 40 cases (64.5%) out of the 62 cases of diffuse large B-cell lymphoma that we studied. The expression rate of p16 was 31.8% in the high risk group and 82.5% in the low risk group of diffuse large B-cell lymphoma. The expression rate of p16 in the fatal cases was 12.5%. For our results, the loss of a p16 expression in diffuse large B-cell lymphoma was noted, and especially in the high risk group (P=0.04).
Conclusion:
The loss of p16 appeared to be involved in the genesis or progression of diffuse large B-cell lymphoma. In addition, the loss of the p16 expression may play important roles for patients' progression into the high risk group and it may cause more mortality for those patients with diffuse large B-cell lymphoma. Deranged expressions and loss of the p16 expression may play an important role in the pathogenesis of diffuse large B-cell lymphoma.
REFERENCES
1). Ko YH., Kim CW., Park CS, et al. REAL classfication of malignant lymphomas in the Republic of Korea: incidence of recently recognized entities and changes in clinicopathologic features. Hematolymphoreticu-lar Study Group of the Korean Society of Pathologists. Revised European-American lymphoma. Cancer. 1998. 83:806–12.
2). Ioachim HL. The revised European-American classification of lymphoid neoplasms. A belated commentary. Cancer. 1996. 78:4–9.

3). A predictive model for aggressive non-Hodgkin's lymphoma. The international non-Hodgkin's lymphoma prognostic factors project. N Engl J Med. 1993. 329:987–94.
4). Peter M., Herskowitz I. Joining the complex: cyclin-dependent kinase inhibitory proteins and the cell cycle. Cell. 1994. 79:181–4.

5). Ralhan R., Mathew R., Arora S., Bahl R., Shukla NK., Mathur M. Frequent alterations in the expression of tumor suppressor genes p16INK4A and pRb in esophageal squamous cell carcinoma in the Indian population. J Cancer Res Clin Oncol. 2000. 126:655–60.
6). Zhang S., Li F., Shi Y, et al. The relationship of non-Hodgkin's lymphoma with P16 protein expression. Hua Xi Yi Ke Da Xue Xue Bao. 1996. 27:254–7.
7). Itami M., Takenouchi T., Tamaru J., Harigaya K., Mikata A. Expression of functional molecules in non-Hodgkin's lymphoma. Correlation with bone marrow involvement and serum LDH value. Acta Pathol Jpn. 1991. 41:277–85.
8). Oshima K., Suzumiya J., Sato K., Kanda M., Haraoka S., Kikuchi M. B-cell lymphoma of 708 cases in Japan: incidence rates and clinical prognosis according to the REAL classification. Cancer Lett. 1999. 135:73–81.
9). Cortelazzo S., Rossi A., Oldani E, et al. The modified International Prognostic Index can predict the outcome of localized primary intestinal lymphoma of both extranodal marginal zone B-cell and diffuse large B-cell histologies. Br J Haematol. 2002. 118:218–28.

10). Herman JG., Merlo A., Mao L, et al. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995. 55:4525–30.
11). Dai CY., Furth EE., Mick R, et al. p16(INK4a) expression begins early in human colon neoplasia and correlates inversely with markers of cell proliferation. Gastroenterology. 2000. 119:929–42.

12). Milde-Langosch K., Bamberger AM., Rieck G., Kelp B., Löning T. Overexpression of the p16 cell cycle inhibitor in breast cancer is associated with a more malignant phenotype. Breast Cancer Res Treat. 2001. 67:61–70.

13). Baur AS., Shaw P., Burri N., Delacrétaz F., Bosman FT., Chaubert P. Frequent methylation silencing of p15(INK4b) (MTS2) and p16(INK4a) (MTS1) in B-cell and T-cell lymphomas. Blood. 1999. 94:1773–81.
14). Villuendas R., Sánchez-Beato M., Martinez JC, et al. Loss of p16/INK4A protein expression in non-Hodgkin's lymphomas is a frequent finding associated with tumor progression. Am J Pathol. 1998. 153:887–97.

15). Paik JH., Jeon YK., Park SS, et al. Expression and prognostic implications of cell cycle regulatory molecules, p16, p21, p27, p14 and p53 in germinal centre and non-germinal centre B-like diffuse large B-cell lymphomas. Histopathology. 2005. 47:281–91.

16). Shiozawa E., Takimoto M., Makino R, et al. Hyper-methylation of CpG islands in p16 as a prognostic factor for diffuse large B-cell lymphoma in a high-risk group. Leuk Res. 2006. 30:859–67.

17). Neumeister P., Hoefler G., Beham-Schmid C, et al. Deletion analysis of the p16 tumor suppressor gene in gastrointestinal mucosa-associated lymphoid tissue lymphomas. Gastroenterology. 1997. 112:1871–5.

18). Møller MB., Kania PW., Ino T, et al. Frequent disruption of the RB1 pathway in diffuse large B cell lymphoma: prognostic significance of E2F-1 and p16INK4A. Leukemia. 2000. 14:898–904.

19). Rocco A., Schandl L., Nardone G, et al. Loss of expression of tumor suppressor p16(INK4) protein in human primary gastric cancer is related to the grade of differentiation. Dig Dis. 2002. 20:102–5.
20). Kondo K., Takahashi Y., Hirose Y, et al. The reduced expression and aberrant methylation of p16(INK4a) in chromate workers with lung cancer. Lung Cancer. 2006. 53:295–302.

21). Chakravarti A., Heydon K., Wu CL, et al. Loss of p16 expression is of prognostic significance in locally advanced prostate cancer: an analysis from the Radiation Therapy Oncology Group protocol 86-10. J Clin Oncol. 2003. 21:3328–34.

22). Kim BN., Yamamoto H., Ikeda K, et al. Methylation and expression of p16INK4 tumor suppressor gene in primary colorectal cancer tissues. Int J Oncol. 2005. 26:1217–26.

23). Masumoto N., Fujii T., Ishikawa M, et al. P16 over-expression and human papillomavirus infection in small cell carcinoma of the uterine cervix. Hum Pathol. 2003. 34:778–83.
Fig. 1
(A, B) The microscopic morphology of diffuse large B-cell lymphoma. The tumor is composed of medium-sized to large lymphoid cells with oval to round, vesicular nuclei with fine chromatin and membrane bound nucleoli (H&E stain, A: ×200, B: ×400). (C, D) Immunostaining for p16 in low-risk group (C) and in the case of high-risk group (D) of diffuse large B-cell lymphoma. p16 is strongly expressed in the nuclei of low-risk group (C), whereas high-risk group diffuse large B-cell lymphoma cells are weakly expressed in the nuclei (D).

Fig. 2
Kaplan-Meier survival curve stratified according to the extent of p16. When the expression of p16 is stratified as 10% or more (n=40) and below 10% (n=22), the survival curve of p16 shows no significance.
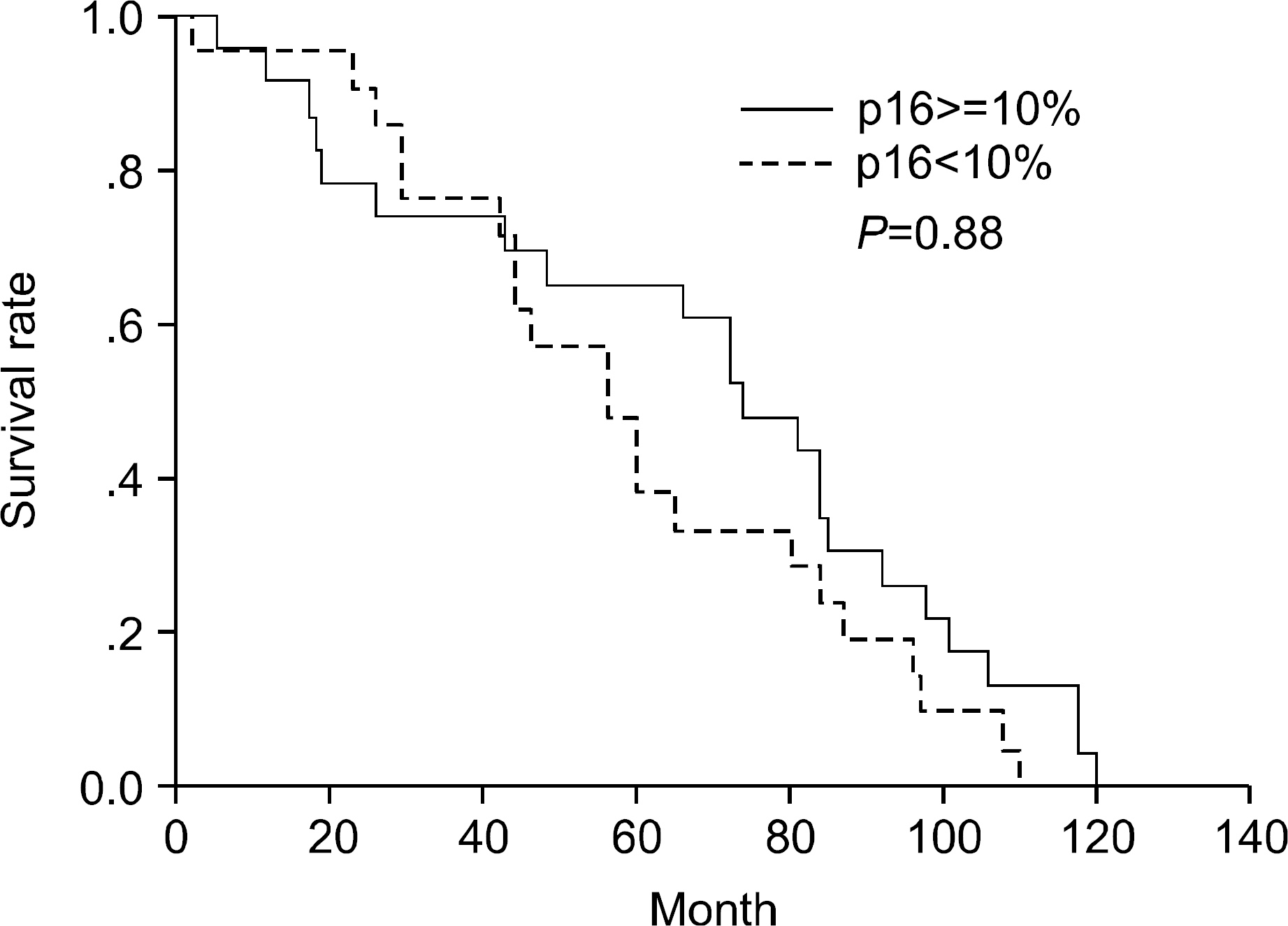
Table 1.
Clinical data of 62 cases with diffuse large B-ce lymphoma
Table 2.
Patient characteristics of high and low risk group according to the International Prognostic Index




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download