Abstract
Background:
Melphalan is frequently used in conditioning regimens prior to autologous stem cell transplantation. The pharmacokinetics of melphalan have shown great interindividual variations. This study aims to investigate the pharmacokinetics of melphalan and to study those influences on renal function and the clinical outcomes.
Methods:
The pharmacokinetics of melphalan following high dose (100∼140mg/m2) i.v. administration was determined in 11 patients suffering with advanced malignancies and who were undergoing autologous stem cell transplantation. The plasma level of melphalan was assayed by a specific HPLC method.
Results:
The pharmacokinetics parameters (median AUC: 625.2mg/L/min, steady-state volume of distribution (Vdss): 14.87L/m2, total body clearance: 0.2L/min/m2, half life: 55.9 min, and maximal plasma concentration: 7.61mg/L) were similar to those reported in the literature. No relationship between the pharmacokinetics of melphalan and creatinine clearance, the non-hematologic toxicities and relapse was observed. Only Vdss was correlated with myelosuppression (P<0.05).
Go to : 
REFERENCES
1). Sarosy G., Leyland-Jones B., Soochan P., Cheson BD. The systemic administration of intravenous melphalan. J Clin Oncol. 1998. 6:1768–82.

2). Samuels BL., Bitran JD. High-dose intravenous melphalan: a review. J Clin Oncol. 1995. 13:1786–99.

3). Pinguet F., Joulia JM., Martel P., Grosse PY., Astre C., Bressolle F. High-performance liquid chromatographic assay for melphalan in human plasma. Application to pharmacokinetic studies. J Chromatogr B Biomed Appl. 1996. 686:43–9.

4). Pinguet F., Martel P., Fabbro M, et al. Pharmacokinetics of high-dose intravenous melphalan in patients undergoing peripheral blood hematopoietic progenitor-cell transplantation. Anticancer Res. 1997. 17:605–11.
5). Tricot G., Alberts DS., Johnson C, et al. Safety of au-totransplants with high-dose melphalan in renal failure: a pharmacokinetic and toxicity study. Clin Cancer Res. 1996. 2:947–52.
6). Kergueris MF., Milpied N., Moreau P., Parousseau JL., Larousse C. Pharmacokinetics of high-dose melphalan in adults: influence of renal function. Anticancer Res. 1994. 14:2379–82.
7). Cornwell GG 3rd., Pajak TF., Mclntyre OR., Kochwa S., Dosik H. Influence of renal failure on myelosup-pressive effects of melphalan: Cancer and Leukemia Group B experience. Cancer Treat Rep. 1982. 66:475–81.
8). Ballester OF., Tummala R., Janssen WE, et al. High-dose chemotherapy and autologous peripheral blood stem cell transplantation in patients with multiple myeloma and renal insufficiency. Bone Marrow Transplant. 1997. 20:653–6.

9). Tosi P., Zamagni E., Ronconi S, et al. Safety of autologous hematopoietic stem cell transplantation in patients with multiple myeloma and chronic renal failure. Leukemia. 2000. 14:1310–3.

10). Sirohi B., Powles R., Kulkarni S, et al. Glomerular filtration rate prior to high-dose melphalan 200mg/m(2) as a surrogate marker of outcome in patients with myeloma. Br J Cancer. 2001. 85:325–32.
11). McCune JS., Gibbs JP., Slattery JT. Plasma concentration monitoring of busulfan: does it improve clinical outcome? Clin Pharmacokinet. 2000. 39:155–65.
Go to : 
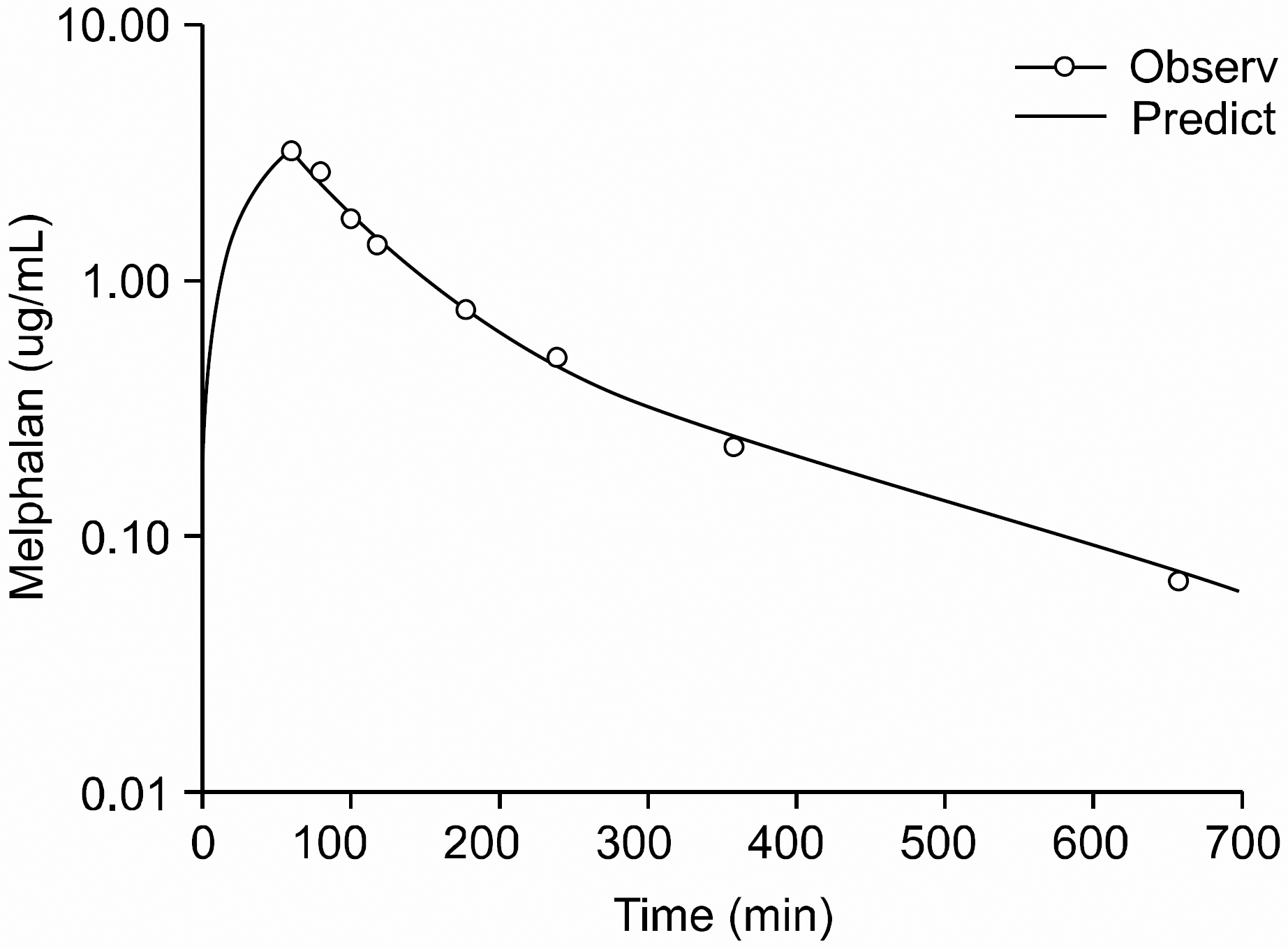 | Fig. 1Representative profile of concentration-time curve in a patient given intravenous administration of melphalan 140mg/m2. |
Table 1.
Conditioning regimens according to diagnosis
| Drugs | Dosing regimen | Days of administration | No. patients (disease) |
|---|---|---|---|
| Cytarabine | 1,500mg/m2 IV q 12 h×6 | Day -6, -5, -4 | |
| Melphalan | 140mg/m2 IV q 24 h×1 | Day -3 | 4 (AML†=3, ALL‡=1) |
| TBI∗ | 1,200cGY×3 | Day -9, -8, -7 | |
| Melphalan | 100mg/m2 IV q 24 h×2 | Day -3, -2 | 6 (MM§) |
| Carmustine | 300mg/m2 IV×1 | Day -7 | |
| Etoposide | 200mg/m2 IV×4 | Day -6, -5, -4, -3 | 1 (NHL∥=1) |
| Cytarabine | 100mg/m2 IV×4 | Day -6, -5, -4, -3 | 1 (NHL =1) |
| Melphalan | 140mg/m2 IV×1 | Day -2 |
Table 2.
Serious adverse events of patients treated wit intravenous melphalan
Table 3.
Transplant outcomes
| Case | ANC∗>500/μL (days) | ? Platelet> 20,000/μL (days) | Current status | DFS† |
|---|---|---|---|---|
| 1 | 8 | 9 | Alive; relapse | 2 months |
| 2 | 9 | 11 | Alive | 17+months |
| 3 | 13 | 17 | Alive | 37+months |
| 4 | 13 | 15 | Alive; relapse | 12 months |
| 5 | 10 | 12 | Alive; relapse | 1.5 months |
| 6 | 12 | 12 | Alive | 2+months ? |
| 7 | 12 | 12 | Alive | 12+months |
| 8 | 14 | 22 | Alive | 16+months |
| 9 | 10 | 10 | Died; relapse | 2 months |
| 10 | 11 | 16 | Alive | 19+months |
| 11 | 12 | 14 | Died; relapse | 2 months |
Table 4.
Pharmacokinetic parameters of melphalan
| Case | Day | Cmax∗ (mg/L) | † (min) t1/2 | AUC‡ (mg/L×min) | § (L/min/m2) ClT | MRT∥ (min) | Vdss¶ (L/m2) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 21.94 | 55.9 | 1,157.2 | 0.119 | 43.2 | 5.15 |
| 2 | 1 | 10.45 | 43.2 | 772.9 | 0.127 | 57.6 | 7.32 |
| 2 | 8.99 | 47.7 | 926.1 | 0.107 | 78.7 | 8.43 | |
| 3 | 1 | 6.81 | 60.2 | 510.5 | 0.191 | 65.3 | 12.49 |
| 2 | 3.09 | 150.2 | 411.1 | 0.235 | 149.5 | 35.12 | |
| 4 | 1 | 3.48 | 46.7 | 347.6 | 0.285 | 75.3 | 21.47 |
| 2 | 7.16 | 71.4 | 493.1 | 0.199 | 62.9 | 12.55 | |
| 5 | 1 | 11.31 | 93.0 | 711.6 | 0.128 | 79.8 | 10.18 |
| 2 | 5.46 | 72.0 | 445.8 | 0.220 | 75.9 | 16.67 | |
| 6 | 1 | 12.97 | 22.7 | 859.9 | 0.116 | 48.6 | 5.64 |
| 2 | 9.7 | 55.5 | 919.2 | 0.107 | 76.5 | 8.23 | |
| 7 | 1 | 6.03 | 24.7 | 588.7 | 0.236 | 64.5 | 15.24 |
| 8 | 1 | 7.61 | 63.4 | 736.1 | 0.174 | 87.7 | 15.31 |
| 9 | 1 | 8.69 | 70.4 | 661.2 | 0.200 | 71.2 | 14.22 |
| 10 | 1 | 6.73 | 38.5 | 625.2 | 0.218 | 68.1 | 14.87 |
| 11 | 1 | 4.05 | 43.8 | 297.4 | 0.330 | 56.7 | 18.71 |
| 2 | 4.06 | 62.8 | 324.8 | 0.304 | 69.4 | 21.07 | |
| Median | 7.61 | 55.9 | 625.2 | 0.200 | 68.2 | 14.87 | |
| Quartile range | 4.40 | 31.3 | 388.7 | 0.117 | 14.8 | 9.13 | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download