Abstract
Background:
Angiogenesis is enhanced in the ischemic tissues after the injection of bone marrow cells (BMCs). However the exact mechanisms for this are not yet fully understood.
Methods:
A unilateral ischemic limb was surgically induced in mice and then BMCs were injected into the ischemic area. We measured the capillary/muscle ratio. Fluorescence-labeled BMCs were injected into the ischemic tissues and then the locations of the cells were examined by using a confocal microscope. Recruitment of bone marrow-derived cells into the ischemic tissue was examined in a sex-mismatched bone marrow transplantation (BMT) setting by identifying the Y chromosome with using the FISH technique. The expressions of VEGF, MMP-9, SDF-1 and CXCR-4 were measured by Western blot analysis.
Results:
The capillary/muscle ratio was more increased in the BMC-injected group than in the control group (P<0.05). Florescence-labeled BMCs, which had been directly injected into ischemic tissue, were not detected in the tissue. In the sex-mismatched bone marrow transplantation models, the ischemic tissues of the BMC-injected group recruited a much greater number of Y chromosome-positive bone marrow-derived cells, as compared to the control group. The expressions of VEGF and MMP-9 were increased after injection of BMCs. SDF-1 was expressed on the seventh day in the BMC-injected group and CXCR-4 was highly expressed until 12 weeks in the BMC-injected group.
REFERENCES
1). Grunewald M., Avraham I., Dor Y, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006. 124:175–89.

2). Zentilin L., Tafuro S., Zacchigna S, et al. Bone marrow mononuclear cells are recruited to the sites of VEGF-induced neovascularization but are not incorporated into the newly formed vessels. Blood. 2006. 107:3546–54.

3). Ruiz de Almodovar C., Luttun A., Carmeliet P. An SDF-1 trap for myeloid cells stimulates angiogenesis. Cell. 2006. 124:18–21.

4). Jin DK., Shido K., Kopp HG, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006. 12:557–67.

5). Abbott JD., Huang Y., Liu D., Hickey R., Krause DS., Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004. 110:3300–5.
6). Peled A., Petit I., Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999. 283:845–8.

7). Sipkins DA., Wei X., Wu JW, et al. In vivo imaging of specialized bone marrow endothelial microdoma-ins for tumour engraftment. Nature. 2005. 435:969–73.

8). Shintani S., Murohara T., Ikeda H, et al. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation. 2001. 103:897–903.

9). Heissig B., Hattori K., Dias S, et al. Recruitment of stem and progenitor cells from th bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002. 109:625–37.
10). Asahara T., Masuda H., Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999. 85:221–8.

11). Ribatti D. The involvement of endothelial progenitor cells in tumor angiogenesis. J Cell Mol Med. 2004. 8:294–300.

12). Gunsilius E., Duba HC., Petzer AL, et al. Evidence from a leukaemia model for maintenance of vascular endothelium by bone-marrow-derived endothelial cells. Lancet. 2000. 355:1688–91.

13). Fuchs S., Kornowski R., Weisz G, et al. Safety and feasibility of transendocardial autologous bone marrow cell transplantation in patients with advanced heart disease. Am J Cardiol. 2006. 97:823–9.

14). Tse HF., Kwong YL., Chan JK., Lo G., Ho CL., Lau CP. Angiogenesis in ischemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003. 361:47–9.
15). Rafii S., Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003. 9:702–12.

16). Luttun A., Tjwa M., Moons L, et al. Revascularization of ischemic tissues by PIGF treatment, and inhibition of tumor angiogenesis, arthritis and artherosclerosis by anti-Flt1. Nat Med. 2002. 8:831–40.
17). Colson YL., Wren SM., Schuchert MJ, et al. A nonlethal conditioning approach to achieve durable multilineage mixed chimerism and tolerance across major, minor, and hematopoietic histocompatibility barriers. J Immunol. 1995. 155:4179–88.
18). Jin DK., Shido K., Kopp HG., Petit I, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006. 12:557–67.

19). O'Neill TJ., Wamhoff BR., Owens GK., Skalak TC. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without trans-differentiation into endothelial cells. Circ Res. 2005. 97:1027–35.
Fig. 1
The injection of bone marrow cells into the ischemic limb increased angiogenesis. Ischemia was induced in the hind limb of mouse, and bone marrow cells injected into the lesion 10 days after the induction. (A) The capillary/muscle ratio was more increased in the bone marrow cell-injected group than in the control group (P<0.05). (B) Photomicroscopic examination findings of the lesions which were stained with alkaline phosphatase also showed highly increased angiogenesis in the bone marrow cell-injected group. Blue spots show the viable endothelial cells at 4 weeks after the induction of ischemia. Maginficati-on, ×200. Control, control group; BM, bone marrow cell-injected group.
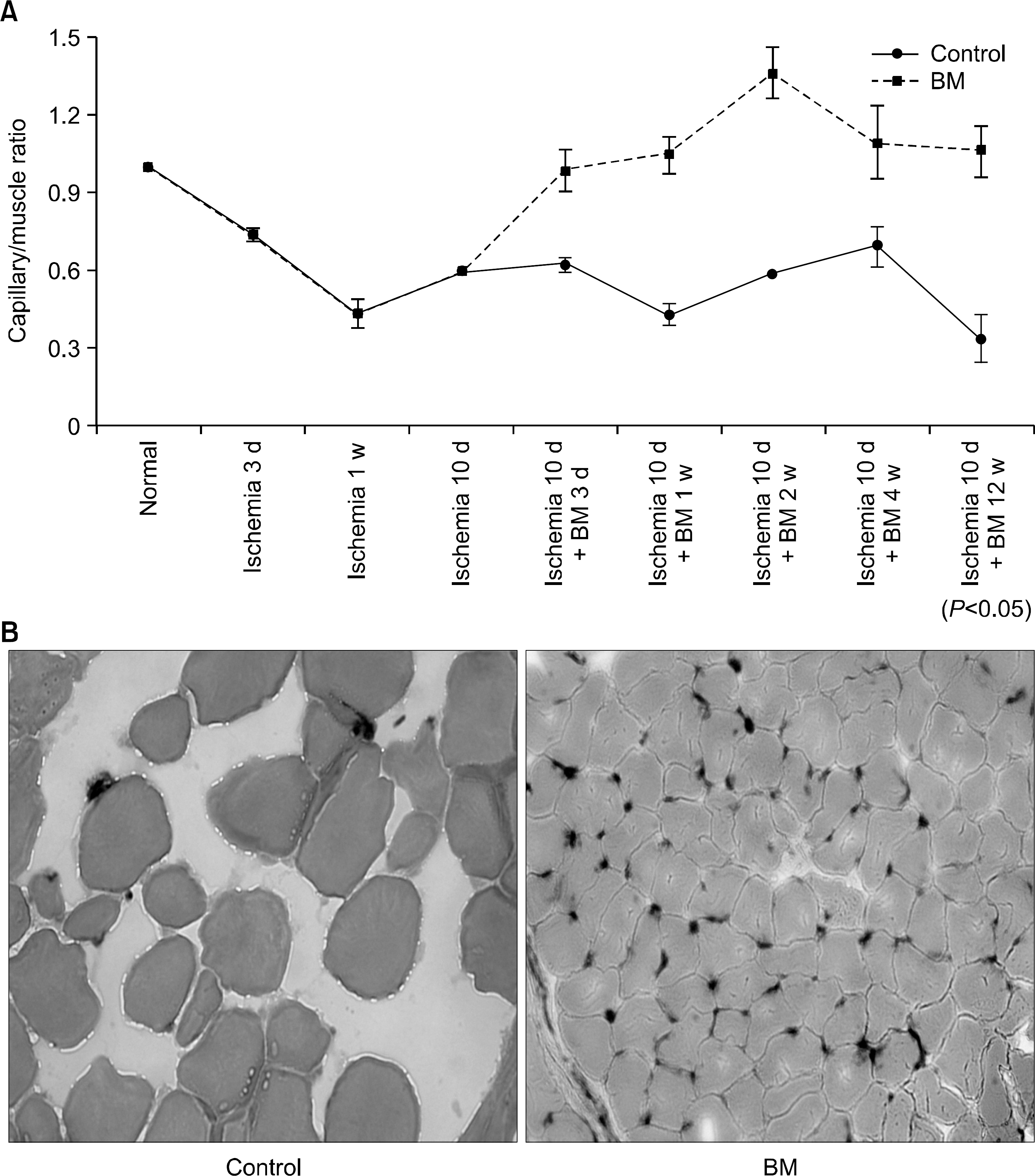
Fig. 2
The Localization of transplanted cells in the ischemic limb. Fluorescence-labeled bone marrow cells were injected into the lesion 10 days after the induction of ischemia and the presence of the cells were examined using confocal laser scanning microscope at 4 weeks after the bone marrow cells injection. The DNA of nucleus was stained with DAPI (arrows, blue). Fluorescence-labeled bone marrow cells were not detected in the ischemic tissues, Magnification, ×200. Abbreviation: M, muscle.
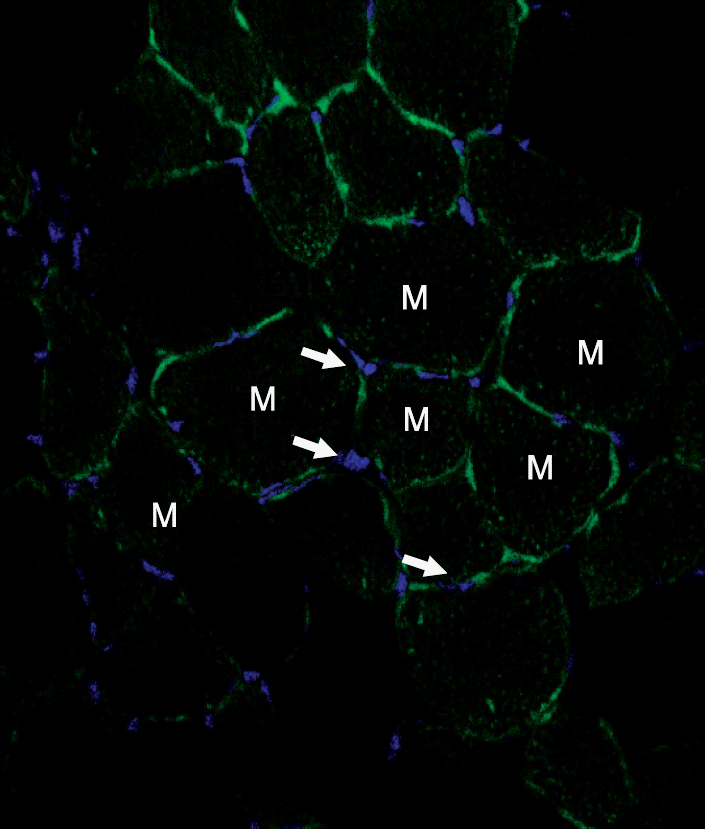
Fig. 3
The increased recruitment of autologous bone marrow cells by injected bone marrow cells. Ischemia was induced in the hind limb of sex-mismatched bone marrow transplanted mouse (A), and bone marrow cells were injected into the lesion 10 days after the ischemia induction (B), and the cells immunoreacitve to Y chromosome were examined 4 weeks after the bone marrow cell-injection using FISH method. Bone marrow cell-transplanted group (B) showed much higher number of Y chromosome-positive bone marrow cells than control group (A), Magnification, ×200.
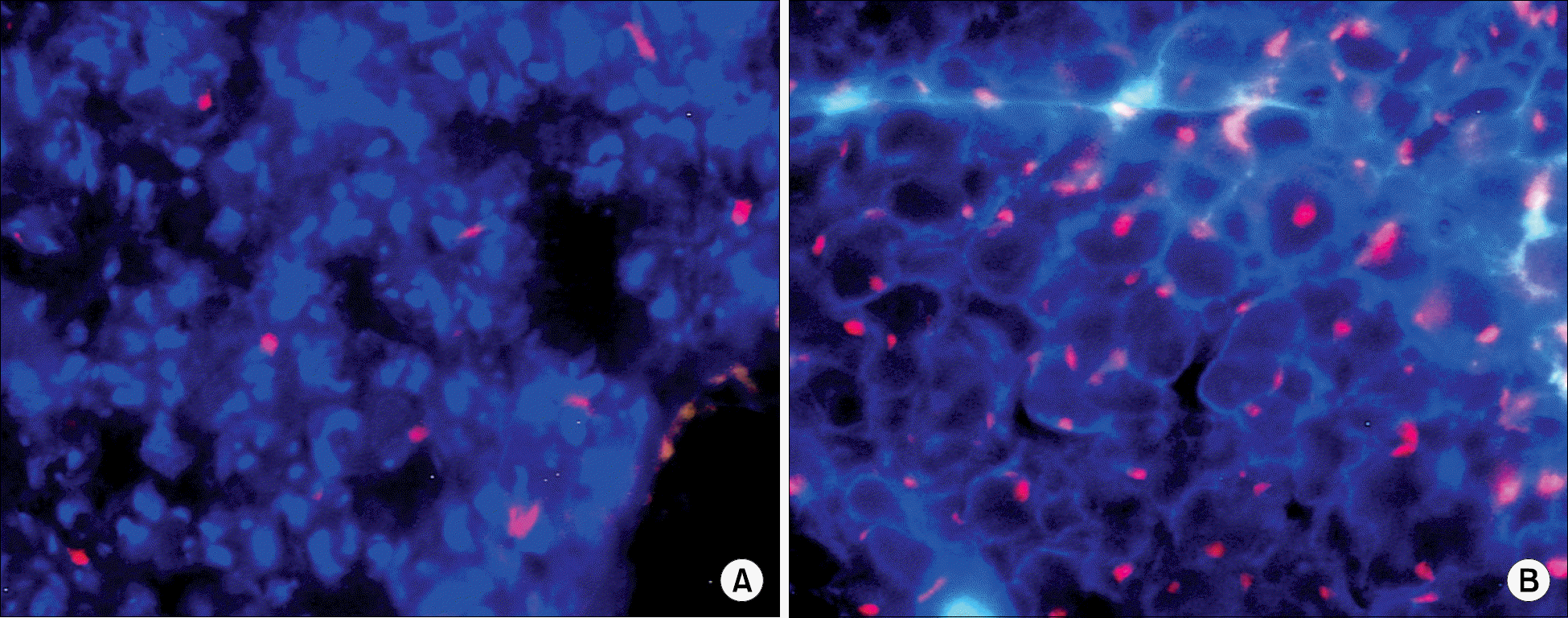
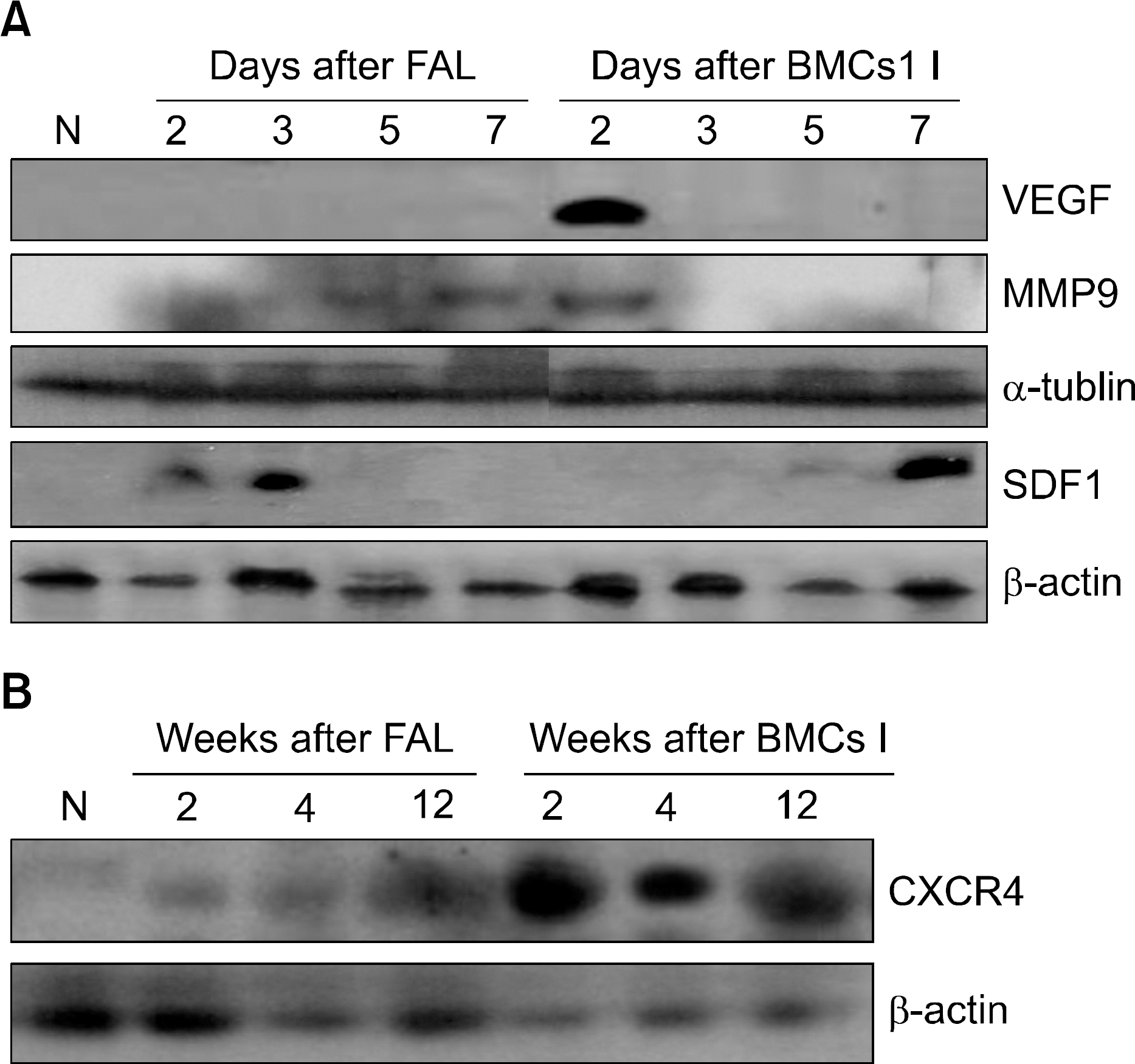
Fig. 4
The expressions of VEGF, MMP-9, SDF-1 and CXCR-4 in the ischemic limb. (A) The expressions of VEGF, MMP-9, and SDF-1 were examined at the indicated times in both ischemic control and bone marrow cell-injected groups using western blot analysis. The expression of VEGF was not detected in the ischemic control group, but it was transiently increased on the second day after the transplantation. The expression of MMP-9 was detected on the fifth and seventh days after the induction of ischemia and on the second day after the injection of bone marrow cells. The expression of SDF-1 was detected on the second and third days in the ischemic control group, and it was increased on the seventh day after the transplantation. Actin and tubulin were used as a loading control. (B) The expression of CXCR-4 was examined at the indicated times in both ischemic control and bone marrow cell-injected groups using western blot analysis. CXCR-4 was increased on second week in the ischemic control group, but it was continuously increased after bone marrow cells injection. Actin was used as a loading control. Abbreviations: N, normal; FAL, femoral artery ligation; BMCs I, bone marrow cells injections.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download