Abstract
Much of the success of hematopoietic stem cell transplantation (HSCT) has been due to the ability to overcome posttransplant complications, by performing HLA genotypically matched or even mismatched sibling donor HSCT or unrelated donor HSCT. Based on the promising results with using vigorously T-cell-depleted high-dose peripheral blood stem cell transplants by the Perugia University group, several methods to eradicate refractory leukemic cells have been employed worldwide. However, there are limitations of the existing data regarding haploidentical HSCT, that is, the small numbers of patients and the heterogeneous patient populations in most of the published series. Also, researchers have not exactly demonstrated the effect of natural killer (NK) cell alloreactivity in various settings of allogeneic HSCT. In reality, haploidentical HSCT is possible without T-cell depletion. However, it isn’t clear whether reduced-intensity HSCT from a haploidentical related donor or a mismatched unrelated donor is feasible. Anyhow, successfully overcoming the major histocompatibility barriers with using related or unrelated donors means that virtually all patients would have an immediately available donor for desperately needed HSCT. The full potential of haploidentical HSCT may be ultimately achieved through a better understanding of the transplant immunology, including the Korean specificity of killer cell immunoglobulin-like receptor polymorphism. Further study and better support from Korean government insurance coverage that would offer HSCT to more patients in need of transplant and cultivating an optimal NK alloreaction without detrimental complications is urgently required.
Go to : 
REFERENCES
1). Gatti RA., Meuwisen HJ., Allen HD., Hong R., Good RA. Immunological reconstitution of sex-linked lym-phopenic immunological deficiency. Lancet. 1968. 2:1366–9.
2). Buckner CD., Epstein RB., Rudolph RH., Clift RA., Storb R., Thomas ED. Allogeneic marrow engraftment following whole body irradiation in a patient with leukemia. Blood. 1970. 35:741–50.

3). Thomas ED., Storb R., Fefer A, et al. Aplastic anemia treated by marrow transplantation. Lancet. 1972. 1:284–9.
4). Thomas ED., Storb R., Clift RA, et al. Bone-marrow transplantation. N Engl J Med. 1975. 292:895–902.

5). Buckner CD., Clift RA., Fefer A, et al. Marrow transplantation for the treatment of acute leukemia using HL-A-identical siblings. Transplant Proc. 1974. 6:365–6.
6). Hansen JA., Clift RA., Thomas ED., Buckner CD., Storb R., Giblett ER. Transplantation of marrow from an unrelated donor to a patient with acute leukemia. N Engl J Med. 1980. 303:565–7.

7). Sierra J., Storer B., Hansen JA, et al. Transplantation of marrow cells from unrelated donors for treatment of high-risk acute leukemia: the effect of leukemic burden, donor HLA-matching, and marrow cell dose. Blood. 1997. 89:4226–35.

8). Kim DW., Min CK., Lee S, et al. Analysis of the process for unrelated stem cell donor search. Korean J Hematol. 2001. 36:18–24.
9). Tseng LH., Lin MT., Hansen JA, et al. Correlation between disparity for the minor histocompatibility antigen HA-1 and the development of acute graft-versus-host disease after allogeneic marrow transplantation. Blood. 1999. 94:2911–4.

10). Akatsuka Y., Warren EH., Gooley TA, et al. Disparity for a newly identified minor histocompatibility antigen, HA-8, correlates with acute graft-versus-host disease after haematopoietic stem cell transplantation from an HLA-identical sibling. Br J Haematol. 2003. 123:671–5.

11). Davies SM., Shu XO., Blazar BR, et al. Unrelated donor bone marrow transplantation: influence of HLA-A and -B incompatibility on outcome. Blood. 1995. 86:1636–42.
12). Petersdorf EW., Gooley T., Malkki M, et al. The biological significance of HLA-DP gene variation in haematopoietic cell transplantation. Br J Haematol. 2001. 112:988–94.

13). Aversa F., Tabilio A., Terenzi A, et al. Successful engraftment of T-cell-depleted haploidentical “Three-loci” incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood. 1994. 84:3948–55.

14). Aversa F., Tabilio A., Velardi A, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from relapsed donors with one fully mismatched HLA haplotype. N Engl J Med. 1998. 339:1186–93.
15). Henslee-Downey PJ., Abhyankar SH., Parrish RS, et al. Use of partially mismatched related donors extends access to allogeneic marrow transplant. Blood. 1997. 89:3864–72.

16). Kim HJ., Min WS., Kim YJ., Kim DW., Lee JW., Kim CC. Haplotype mismatch transplantation using high doses of peripheral blood CD34+cells together with stratified conditioning regimens for high-risk adult acute myeloid leukemia patients: a pilot study in a single Korean institution. Bone Marrow Transplant. 2005. 35:959–64.
17). Beatty PG., Mori M., Milford E, et al. Impact of racial genetic polymorphism on the probability of finding an HLA-matched donor. Transplantation. 1995. 60:778–83.

18). Tiercy JM., Villard J., Roosnek E. Selection of unrelated bone marrow donors by serology, molecular typing and cellular assays. Transplant Immunol. 2002. 10:215–21.

19). Begovich AB., McClure GR., Suraj VC, et al. Polymorphism, recombination, and linkage disequilibrium within the HLA class II region. J Immunol. 1992. 148:249–58.
20). Elsner HA., Blasczyk R. Sequence similarity matching: proposal of a structure-based rating system for bone marrow transplantation. Eur J Immunogenet. 2002. 29:229–36.

21). Petersdorf EW., Longton GM., Anasetti C, et al. Association of HLA-C disparity with graft failure after marrow transplantation from unrelated donors. Blood. 1997. 89:1818–23.

22). Anasetti C., Hansen JA. Effect of HLA incompatibility in marrow transplantation from unrelated and HLA-mismatched related donors. Transfus Sci. 1994. 15:221–30.

23). Flomenberg N., Baxter-Lowe LA., Confer D, et al. Impact of HLA-class I and class II high resolution matching on outcomes of unrelated donor BMT. Blood. 2001. 98:813a.
24). Ho VT., Ki HT., Liney D, et al. HLA-C mismatch is associated with inferior survival after unrelated donor non-myeloablative hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006. 37:845–50.

25). Petersdorf EW., Gooley TA., Anasetti C, et al. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998. 92:3515–20.

26). Kim HJ., Min WS., Eom KS, et al. ATG for GvHD prophylaxis after unrelated peripheral blood stem cell transplantation for AML. Korean J Hematol. 2007. 42:102.
27). Sasazuki T., Juji T., Morishima Y, et al. Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. N Engl J Med. 1998. 339:1177–85.

28). Morishima Y., Sasazuki T., Inoko H, et al. The clinical significance of human leukocyte antigen (HLA) allele compatibility in patients receiving a marrow transplant from serologically HLA-A, HLA-B and HLA-DR matched unrelated donors. Blood. 2002. 99:4200–6.

29). Hurley CK., Baxter-Lowe LA., Begovich AB, et al. The extent of HLA class II allele level disparity in unrelated bone marrow transplantation: analysis of 1259 National Marrow Donor Program donor-recipient pairs. Bone Marrow Transplant. 2000. 25:385–93.

30). Ruggeri L., Capanni M., Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999. 94:333–9.

31). Ruggeri L., Capanni M., Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002. 295:2097–100.

32). Farag SS., Fehniger TA., Ruggeri L., Velardi A., Cali-giuri MA. Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood. 2002. 100:1935–47.

33). Varney MD., Lester S., McCluskey J., Gao X., Tait BD. Matching for HLA DPA1 and DPB1 alleles in unrelated bone marrow transplantation. Hum Immunol. 1999. 60:532–8.

34). Anasetti C., Amos D., Beatty PG, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989. 320:197–204.

35). Szydlo R., Goldman JM., Klein JP, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997. 15:1767–77.

36). McGlave PB., Shu XO., Wen W, et al. Unrelated donor marrow transplantation for chronic myelogenous leukemia: 9 years' experience of the National Marrow Donor Program. Blood. 2000. 95:2219–25.

37). Kim HJ., Choi Y., Jeong HY., Min WS., Kim CC., Kim TG. Killer cell immunoglobulin-like receptor (KIR) analysis in adult Korean patients with acute myeloid leukemia. Korean J Hematol. 2006. 41:139–48.

38). Norman PJ., Stephens HA., Verity DH., Chandanay-ingyong D., Vaughan RW. Distribution of natural killer cell immunoglobulin-like receptor sequences n three ethnic groups. Immunogenetics. 2001. 52:195–205.
39). Crum KA., Logue SE., Curran MD., Middleton D. Development of a PCR-SSOP approach capable of defining the natural killer cell inhibitory receptor (KIR) gene sequence repertoires. Tissue Antigens. 2000. 56:313–26.

40). Niokou D., Spyropoulou-Vlachou M., Darlamitsou A., Stavropoulos-Giokas C. Distribution of killer cell immunoglobulin-like receptors in the Greek population. Hum Immunol. 2003. 64:1167–76.

41). Yin XL., Guo KY., Ma HJ, et al. Killer immunoglobulin-like receptor gene distribution in Guangdong Han population. Di Yi Jun Yi Da Xue Xue Bao. 2004. 24:1416–8.
42). Giebel S., Locatelli F., Lamparelli T, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003. 102:814–9.

43). Malmberg KJ., Schaffer M., Ringden O., Remberger M., Ljunggren HG. KIR-ligand mismatch in allogeneic hematopoietic stem cell transplantation. Mol Immunol. 2005. 42:531–4.

44). De Santis D., Bishara A., Witt CS, et al. Natural killer cell HLA-C epitopes and killer cell immunoglobulin-like receptors both influence outcome of mismatched unrelated donor bone marrow transplants. Tissue Antigens. 2005. 65:519–28.

45). Davies SM., Ruggeri L., DeFor T, et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood. 2002. 100:3825–7.
46). Storb R., Prentice RL., Hansen JA., Thomas ED. Association between HLA-B antigens and acute graft-versus-host disease. Lancet. 1983. 2:816–9.

47). Pawelec G., Muller CA., Haen M., Ehninger G. Chronic graft-versus-host disease and HLA-DR4 in the southern German population. Br J Haematol. 1990. 75:295–6.

48). Kim HJ., Park SJ., Im HW, et al. The association of HLA antigen and GVHD in allogeneic hematopoietic stem cell transplantation with histocompatible sibling donor: a single-center experience in Korea. Int J Hematol. 2002. 76:267–71.
49). Yamasaki S., Ohno Y., Taniguchi S, et al. Allogeneic peripheral blood stem cell transplantation from two-or three-loci-mismatched related donors in adult Japanese patients with high-risk hematologic malignancies. Bone Marrow Transplant. 2004. 33:279–89.
50). Kanda Y., Chiba S., Hirai H, et al. Allogeneic hematopoietic stem cell transplantation from family members other than HLA-identical siblings over the last decade (1991-2000). Blood. 2003. 102:1541–7.

51). Franco A., Terenzi A., Tabilio A, et al. Full haplotype-mismatched hematopoietic stem cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005. 23:3447–54.
52). Drobyski WR., Klein J., Flomenberg N, et al. Superior survival associated with transplantation of matched unrelated versus one-antigen-mismatched unrelated or highly human leukocyte antigen-disparate haploidentical family donor marrow grafts for the treatment of hematologic malignancies: establishing a treatment algorithm for recipients of alternative donor grafts. Blood. 2002. 99:806–14.
53). Ji SQ., Chen HR., Wang HX, et al. G-CSF-primed haploidentical marrow transplantation without ex vivo T cell depletion: an excellent alternative for high-risk leukemia. Bone Marrow Transplant. 2002. 30:861–6.

54). Ji SQ., Chen HR., Yan HM, et al. Anti-CD25 mono-clonal antibody (basiliximab) for prevention of graft-versus-host disease after haploidentical bone marrow transplantation for hematological malignancies. Bone Marrow Transplant. 2005. 36:349–54.

55). Lu DP., Dong L., Wu T, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006. 107:3065–73.

56). Huang X-J., Liu D-H., Liu K-Y, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant. 2006. 38:291–7.

57). Shimazaki C., Ochiai N., Uchida R, et al. Non-T-cell-depleted HLA haploidentical stem cell transplantation in advanced hematologic malignancies based on the feto-maternal michrochimerism. Blood. 2003. 101:3334–6.

58). Handgretinger R., Schumm M., Lang P, et al. Transplantation of megadoses of purified haploidentical stem cells. Ann N Y Acad Sci. 1999. 872:351–61.

59). Teshima T., Matsuo K., Matsue K, et al. Impact of human leucocyte antigen mismatch on graft-versus host disease and graft failure after reduced intensity conditioning allogeneic haematopoietic stem cell transplantation from related donors. Br J Haematol. 2005. 130:575–87.
60). Dey BR., Spitzer TR. Current status of haploidentical stem cell transplantation. Br J Haematol. 2006. 135:423–37.

61). Kröger N., Zabelina T., Krüger W, et al. In vivo T cell depletion with pretransplant antithymocyte globulin reduces graft-versus-host disease without increasing relapse in good risk myeloid leukemia patients after stem cell transplantation from matched related donors. Bone Marrow Transplant. 2002. 29:683–9.

62). Bacigalupo A., Lamparelli T., Bruzzi P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood. 2001. 98:2942–7.

63). Deeg HJ., Storer BE., Boeckh M, et al. Reduced incidence of acute and chronic graft-versus-host disease with the addition of thymoglobulin to a targeted busulfan/cyclophosphamide regimen. Biol Blood Marrow Transplant. 2006. 12:573–84.

64). Sedlacek P., Formankova R., Keslova P, et al. Low mortality of children undergoing hematopoietic stem cell transplantation from 7 to 8/10 human leukocyte antigen allele-matched unrelated donors with the use of antithymocyte globulin. Bone Marrow Transplant. 2006. 38:745–50.

65). van Rood JJ., Loberiza FR Jr., Zhang MJ, et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002. 99:1572–7.

66). Cho BS., Choi HB., Kim HJ., Min WS., Kim CC., Kim TG. Typing by nested PCR-SSP approach raises a question about the feasibility of using this technique for detecting feto-maternal microchimerism. Leukemia. 2006. 20:896–8.

67). Aversa F., Terenzi A., Carotti A, et al. Improved outcome with T-cell-depleted bone marrow transplantation for acute leukemia. J Clin Oncol. 1999. 17:1545–50.

68). Rachamim N., Gan J., Segall H, et al. Tolerance induction by “Megadose” hematopoietic transplants. Transplantation. 1998. 65:1386–93.

69). Martelli MF., Reisner Y. Haploidentical ‘megadose' CD34+cell transplants for patients with acute leukemia. Leukemia. 2002. 16:404–5.
70). Kim HJ., Min WS., Park YH, et al. Megadose CD34+ hemopoietic stem cell transplantation for patients with high risk acute myeloid leukemia who have no HLA matched donor-A pilot study of a full haplotype mismatch transplantation. Korean J Med. 2003. 65:81–9.
71). Kim HJ., Min WS., Choi SM, et al. Haplotype mismatch transplantation using high-dose CD34+cells with stratified new conditioning regimens in patients with acute myeloid leukemia. Korean J Hematol. 2003. 38:221–7.
72). Lee ST., Jang JH., Cheong JW, et al. Treatment of high-risk acute myelogenous leukaemia by myeloablative chemoradiotherapy followed by co-infusion of T cell-depleted haematopoietic stem cells and culture-expanded marrow mesenchymal stem cells from a related donor with one fully mismatched human leucocyte antigen haplotype. Br J Haematol. 2002. 118:1128–31.
73). Whang DH., Park H., Yoon JA., Park MH. Haplotype analysis of killer cell immunoglobulin-like receptor genes in 77 Korean families. Hum Immunol. 2005. 66:146–54.

74). Petersdorf EW., Kollman C., Hurley CK, et al. Effect of HLA class II gene disparity on ethical outcome in unrelated donor hematopoietic cell transplantation for chronic myeloid leukemia: the US National Marrow Donor Program Experience. Blood. 2001. 98:2922–9.
75). Albi N., Ruggeri L., Aversa F, et al. Natural killer (NK) cell function and antileukemic activity of a large population of CD3+/CD8+T-cells expressing NK receptors for major histocompatibility complex class I after “three-loci” HLA-incompatible bone marrow transplantation. Blood. 1996. 87:3993–4000.
76). Rhee CK., Rhee JH., Kim HJ, et al. T-cell lymphoproliferative disorder following a full haplotype-mismatched haematopoietic stem cell transplant in a patient with acute myeloid leukaemia. Bone Marrow Transplant. 2005. 36:461–3.

77). Huang XJ., Liu DH., Liu KY., Xu LP., Chen H., Han W. Donor lymphocyte infusion for the treatment of leukemia relapse after HLA-mismatched/haploiden-tical T-cell-replete hematopoietic stem cell transplantation. Haematologica. 2007. 92:414–7.

78). Klingebiel T., Handgretinger R., Lang P., Bader P., Niethammer D. Haploidentical transplantation for acute lymphoblastic leukemia in childhood. Blood Rev. 2004. 18:181–92.

79). Douek DC., Vescio RA., Betts MR, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000. 355:1875–81.

81). Andre-Schmutz I., Bonhomme D., Yates F, et al. IL-7 effect on immunological reconstitution after HSCT depends on MHC incompatibility. Br J Haematol. 2004. 126:844–51.
82). Min D., Taylor PA., Panoskaltsis-Mortari A, et al. Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood. 2002. 99:4592–600.

83). Alpdogan O., Hubbard VM., Smith OM, et al. Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood. 2006. 107:2453–60.

84). Fichcer JC., Ottinger H., Ferencik S, et al. Relevance of C1 and C2 Epitopes for Hemopoietic Stem Cell Transplantation: role for sequential acquisition of HLA-C-Specific inhibitory killer ig-like receptor. J Immunol. 2007. 178:3918–23.

Go to : 
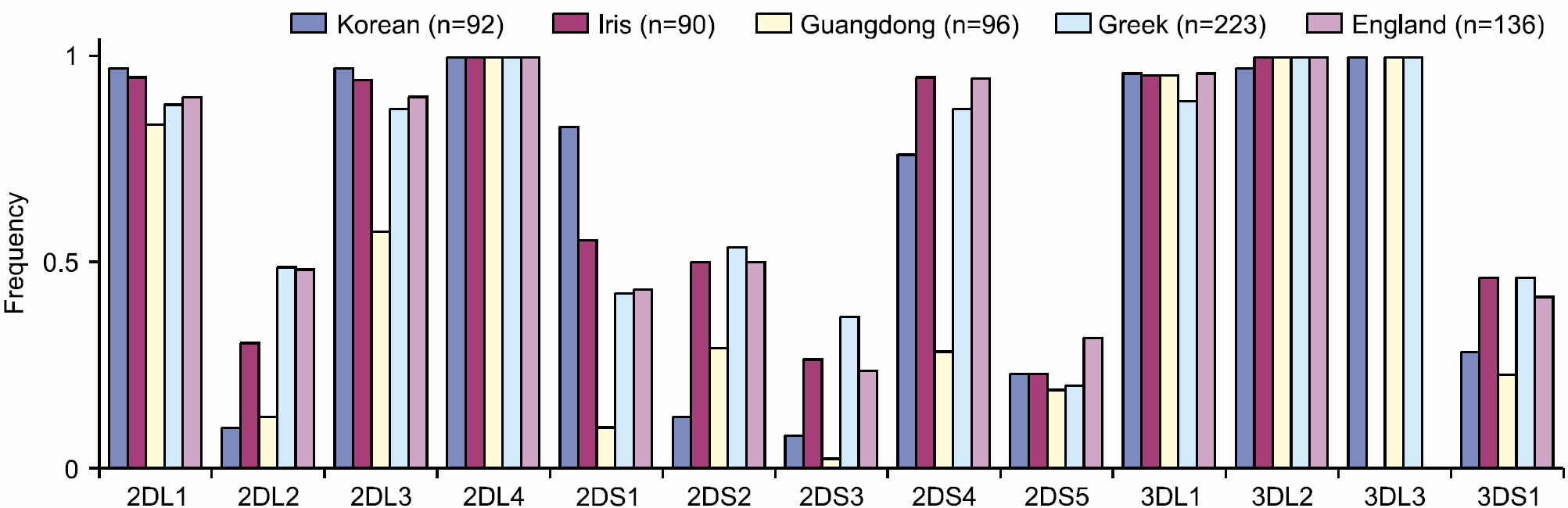 | Fig. 2Gene frequencies of killer cell immunoglobulin-like receptors in different races.37-41)
|
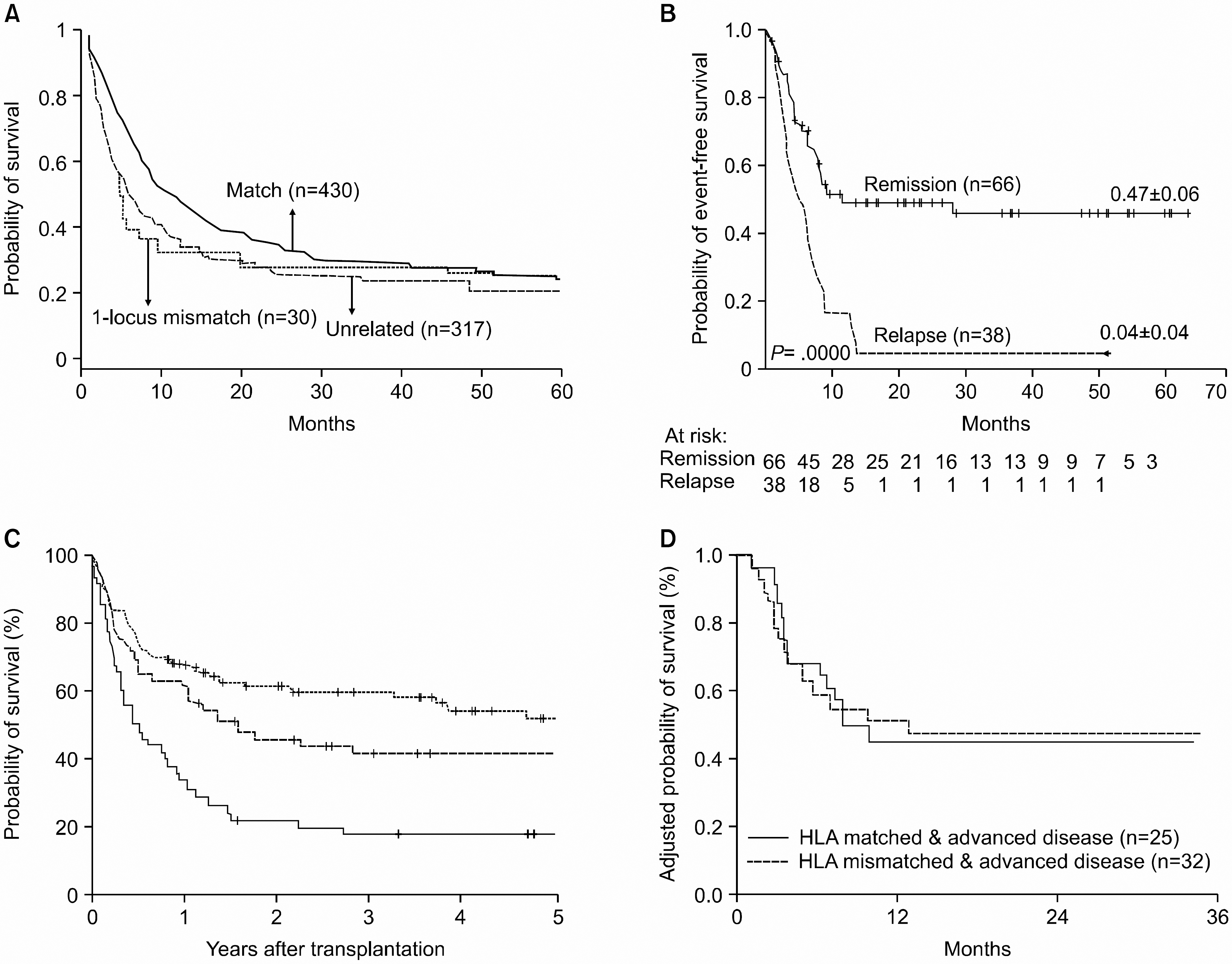 | Fig. 3Comparison of international reports after HLA mismatched donor hematopoietic stem cell transplantation. (A) Overall survival using HLA matched sibling, 1-locus mismatched sibling, and HLA matched unrelated donors in Japan.50) (B) Probability of event-free survival in patients with acute leukemia, either in remission or in relapse from Perugia university, Italy.51) (C) Probability of survival in recipients of matched unrelated (dotted line), mismatched unrelated (dashed line), and haploidentical (solid line) marrow grafts from USA.52) (D) Probability of overall survival HLA-identical or -mismatched related transplantation for patients diagnosed with advanced disease in China.55)
|
Table 1.
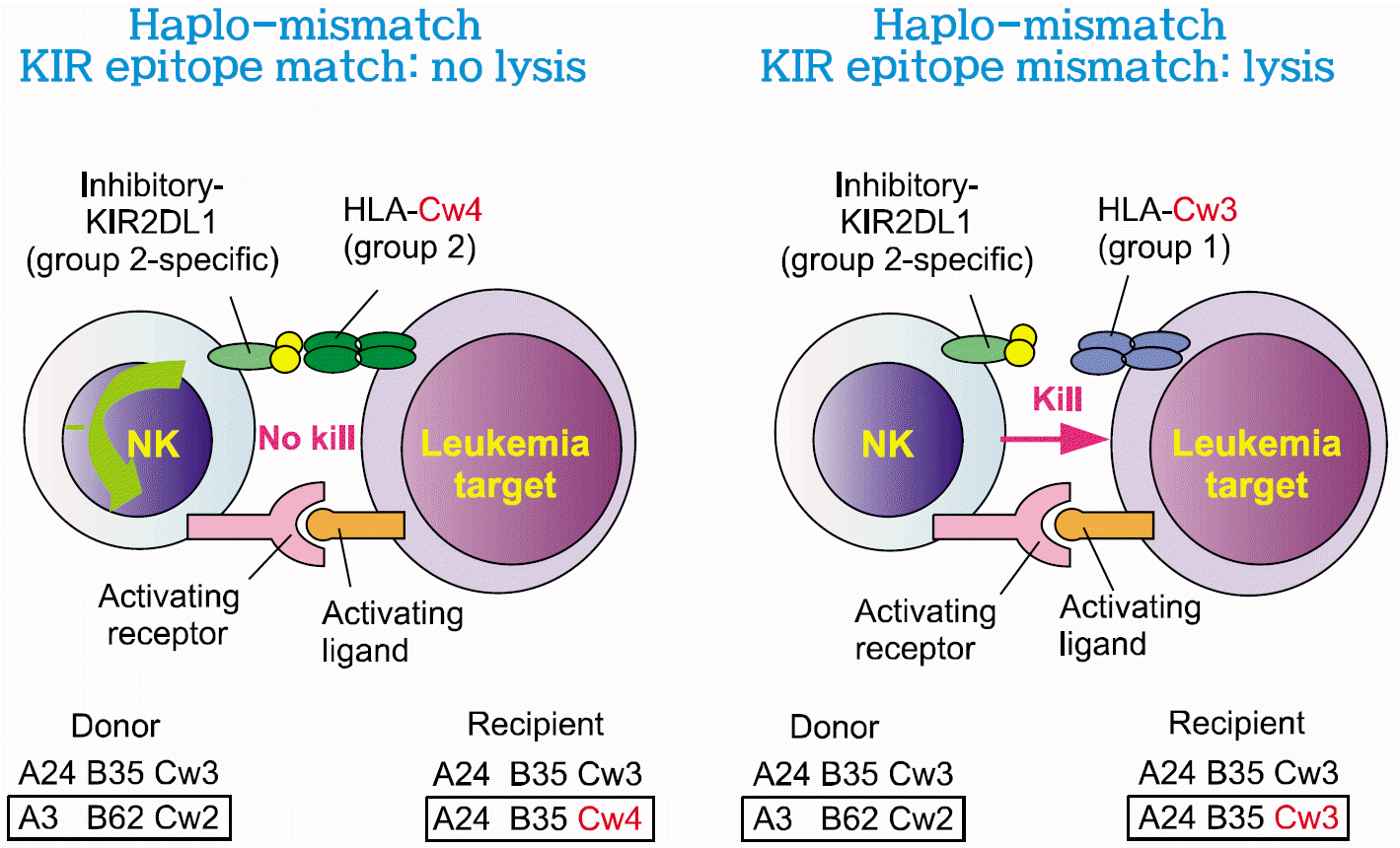
Donor versus recipient natural killer cell alloreactivity in HLA mismatched transplants is determined by the presence or absence of disparity between HLA class I alleles and specific killer cell immunoglobulin-like receptors (KIR). For example, donor natural killer cell expresses KIR 2DL1 and is blocked by HLA-C group 2 alleles, -Cw2 on donor cells and -Cw4 on recipient cells, as shown in the Fig. 1.32)
Table 2.
Different methods of HLA-mismatched related donor hematopoietic stem cell transplantation




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download