Abstract
Background:
The use of non-myeloablative stem cell transplantation (NST) has recently been increasing for treating the patients who cannot tolerate ablative hematopoietic stem cell transplantation (HSCT). Although graft-versus-host disease (GVHD) is one of the greatest problems in HSCT, the clinical effect of GVHD following NST is not clear. We undertook this study to evaluate the clinical manifestations of GVHD and the outcomes after NST.
Methods:
From October 2000 to October 2004, 61 patients underwent NST with a fludarabine-based conditioning regimen. The cumulative incidence of GVHD and the survival rates were obtained from the Kaplan-Meier curves.
Results:
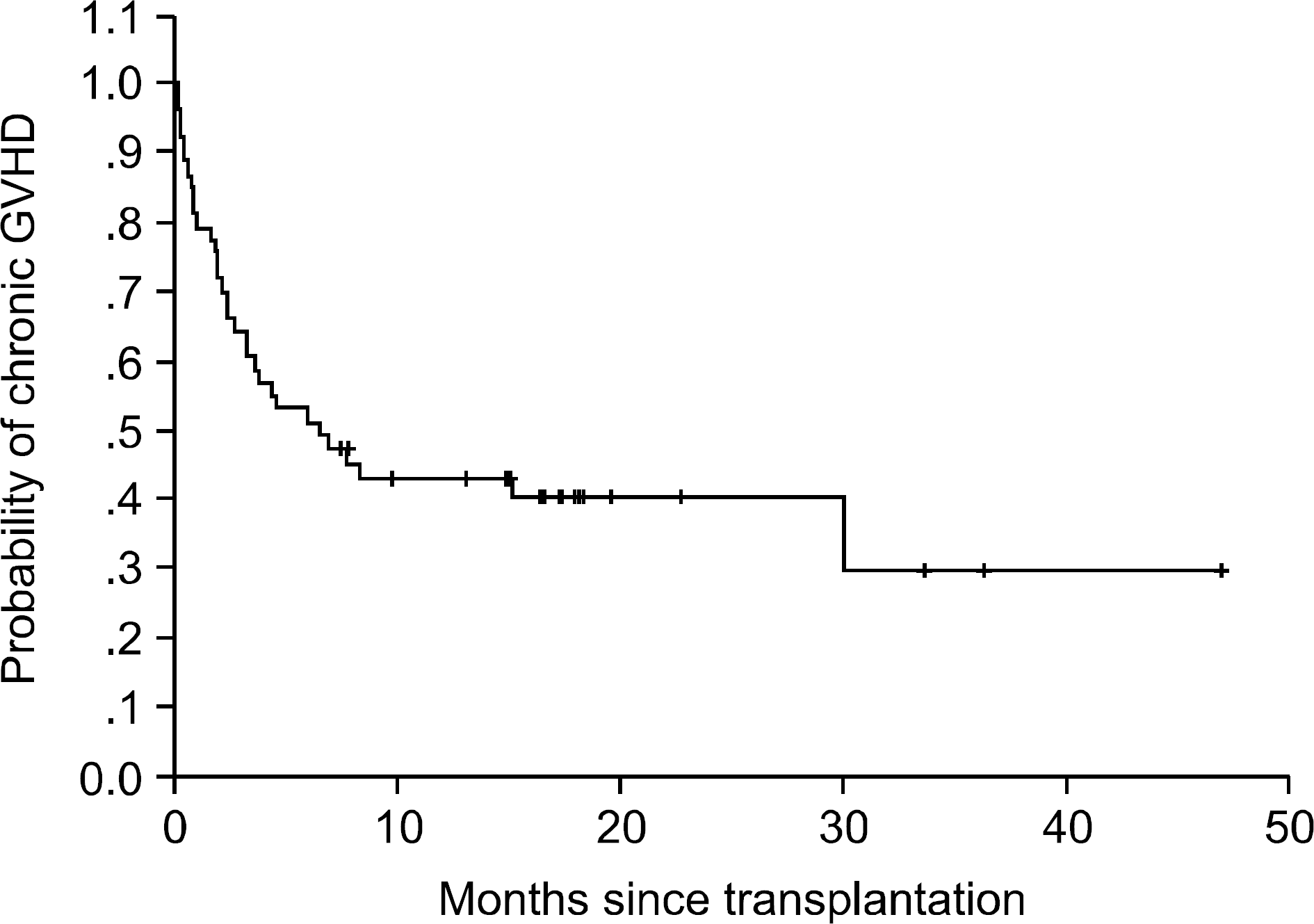
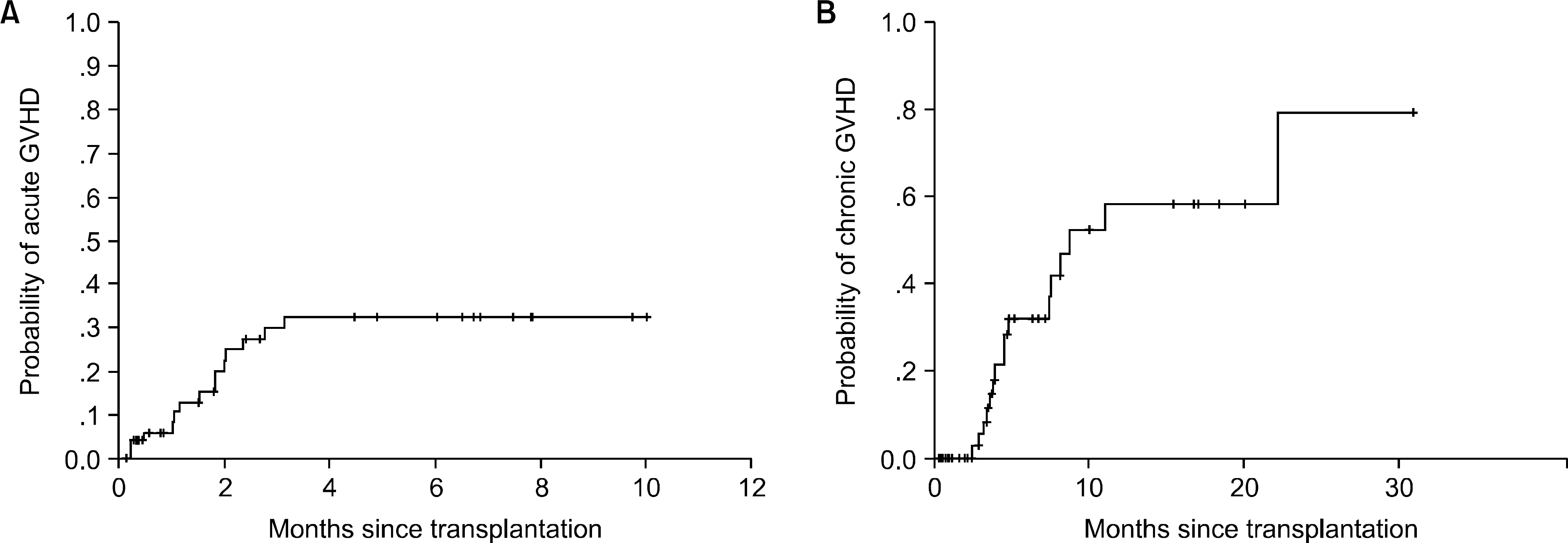
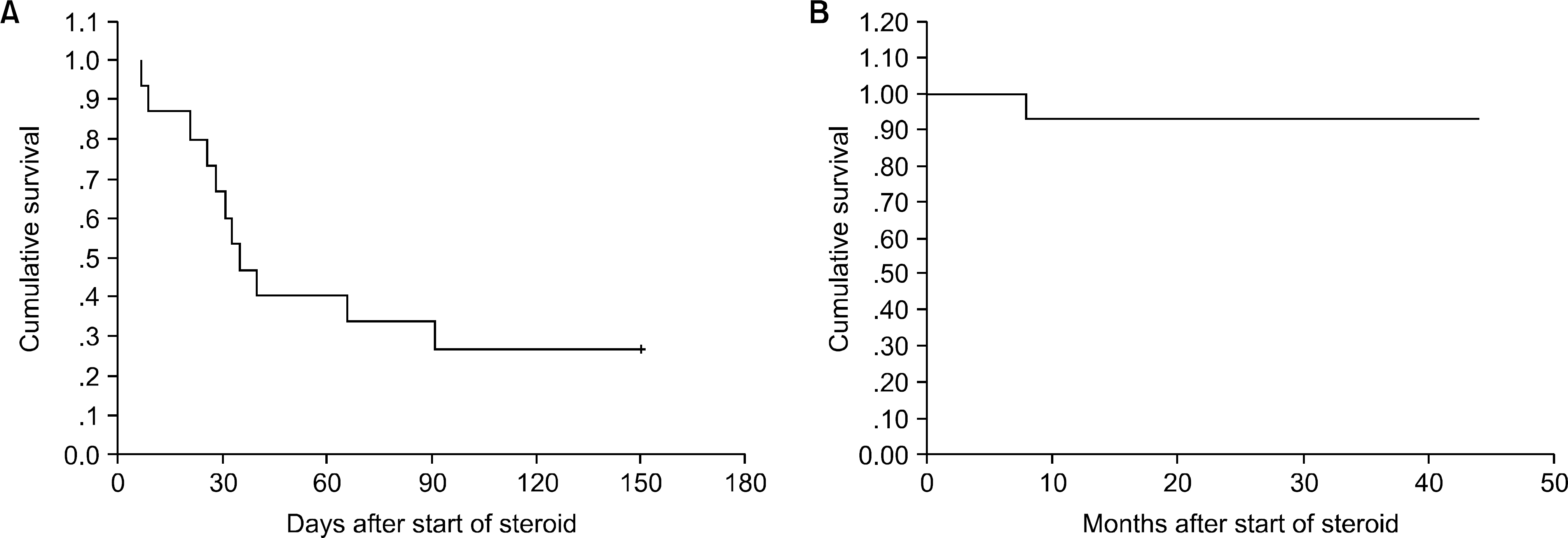
With a median follow-up of 195 days, the estimate for overall three-year survival was 32%. The cumulative incidences of grades II~IV acute GVHD and chronic GVHD were 33% (18/53) and 78% (29/37), respectively. The response rates for acute and chronic GVHD were 33% and 89%, respectively. The survival rates of patients with acute and chronic GVHD were 27% and 89%, respectively. The median survival time was 6.5 months
Go to : 
REFERENCES
1). Weiden PL., Flournoy N., Thomas ED, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979. 300:1068–73.

2). Weiden PL., Sullivan KM., Flournoy N, et al. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981. 304:1529–33.
3). Marijt WA., Heemskerk MH., Kloosterboer FM, et al. Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia. Proc Natl Acad Sci USA. 2003. 100:2742–7.

4). Spitzer TR., McAfee S., Sackstein R, et al. Intentional induction of mixed chimerism and achievement of antitumor responses after nonmyeloablative conditioning therapy and HLA-matched donor bone marrow transplantation for refractory hematologic malignancies. Biol Blood Marrow Transplant. 2000. 6:309–20.

5). Collins RH Jr., Shpilberg O., Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997. 15:433–44.

6). Slavin S., Nagler A., Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998. 91:756–63.

7). Childs R., Chernoff A., Contentin N, et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med. 2000. 343:750–8.

8). McSweeney PA., Niederwieser D., Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001. 97:3390–400.

9). Hill GR., Crawford JM., Cooke KR, et al. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997. 90:3204–13.

10). Hill GR., Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000. 95:2754–9.

11). Cooke KR., Hill GR., Crawford JM, et al. Tumor necrosis factor-alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Invest. 1998. 102:1882–91.
12). McSweenney PA., Niederwieser D., Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dosecytotoxic therapy with graft-versus-tumor effects. Blood. 2001. 97:3390–400.
13). Childs R., Clave E., Contentin N, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999. 94:3234–41.

14). Sullivan KM., Agura E., Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hema-tol. 1991. 28:250–9.
15). Przepiorka D., Weisdorf D., Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995. 15:825–8.
16). Mielcarek M., Martin PJ., Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003. 102:756–62.

17). Sorror ML., Maris MB., Storer B, et al. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after non-myeloablative and myeloablative conditioning: influence of pretransplantation comorbidities. Blood. 2004. 104:961–8.

18). Kim DH., Sohn SK., Baek JH, et al. Retrospective multicenter study of allogeneic peripheral blood stem cell transplantation followed by reduced- intensity conditioning or conventional myeloablative regimen. Acta Haematol. 2005. 113:220–7.
19). Alyea EP., Kim HT., Ho V, et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005. 105:1810–4.

20). Oh H., Loberiza FR Jr., Zhang MJ, et al. Comparison of graft-versus-host-disease and survival after HLA-identical sibling bone marrow transplantation in ethnic populations. Blood. 2005. 105:1408–16.

21). Hong DS., Park SK., Kim HJ, et al. The study of frequency, predictive factors and clinical outcome of graft-versus. Korean J Hematopoietic Stem Cell Trans-planstation. 1999. 4:59–72.
22). Sung WJ., Moon JH., Jeon SB, et al. Single center experience of allogeneic peripheral blood stem cell transplantation for acute myeloid leukemia. Korean J Hematol. 2004. 39:16–22.
Go to : 
Table 1.
Patient characteristics
Table 2.
Diseases of patients who received NST




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download