Abstract
Background:
Several attempts have been made to expand human NK cells from peripheral blood mononuclear cells (PBMCs). This study examined the selective expansion of NK cells using interleukin 2 (IL-2) plus the K562 cell line, the expression of the NK cell receptors, and the cytotoxic activity.
Methods:
The PBMCs from seven healthy volunteers were cultured in a medium containing the IL-2 plus the K562 cell line for 14 days. The expression of the activating and inhibitory receptors on the resting NK cells and the 72 hr-expanded NK cells were analyzed. A flow cytometric cytotoxic assay was used to determined the killing activity of the non-expanded NK cells and the 7 day-expanded NK cells against the K562 target cells.
Results:
The NK cells from PBMCs expanded 4.5-fold after 7 days, and contained 56.5% CD3-CD56+ cells. The IL-2 or IL-2 plus K562 increased the expression levels of CD158b (MFI, mean florescence intensity), CD158e1/e2 (MFI), and NKp44 (MFI), while it decreased the expression levels of NKp30 (%), CD16 (MFI), and 2B4 (MFI). The non-expanded NK cells lysed 9.0% and 27.6% of the K562 target cells in the 1:1 and 5:1 effector and target ratio, respectively, and the 7-day expanded NK cells lysed 36.9% and 57.2% of the K562 target cells, respectively.
Conclusion:
The selective expansion of CD3-CD56+ NK cells occurred only during 7 days of culture. IL-2 or IL-2 plus the K562 cells altered the expression of various activating and inhibitory receptors of NK cells, and the cytotoxicity of the expanded NK cells was higher than in the non-expanded cells.
REFERENCES
1). Kim JS., Lee WK., Suh JS, et al. T and B cell changes with aging. Korean J Lab Medicine. 2001. 21:135–40.
2). Lanier LL., Corliss B., Phillips JH. Arousal and inhibition of human NK cells. Immunol Rev. 1997. 155:145–54.

3). Domzig W., Stadler BM., Herberman RB. Interleukin 2 dependence of human natural killer (NK) cell activity. J Immunol. 1983. 130:1970–3.
4). Burns LJ., Weisdorf DJ., DeFor TE, et al. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant. 2003. 32:177–86.

5). Harada H., Saijo K., Watanabe S, et al. Selective expansion of human natural killer cells from peripheral blood mononuclear cells by the cell line, HFWT. Jpn J Cancer Res. 2002. 93:313–9.

6). Perussia B., Ramoni C., Anegon I., Cuturi MC., Faust J., Trinchieri G. Preferential proliferation of natural killer cells among peripheral blood mononuclear cells cocultured with B lymphoblastoid cell lines. Nat Immun Cell Growth Regul. 1987. 6:171–88.
7). Miller JS., Oelkers S., Verfaillie C., McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood. 1992. 80:2221–9.

8). Carlens S., Gilljam M., Chambers BJ, et al. A new method for in vitro expansion of cytotoxic human CD3-CD56+ natural killer cells. Hum Immunol. 2001. 62:1092–8.

9). Godoy-Ramirez K., Franck K., Gaines H. A novel method for the simultaneous assessment of natural killer cell conjugate and formation and cytotoxicity at the single-cell level by multi-parameter flow cytometry. J Immunol Methods. 2000. 239:35–44.
10). Farag SS., Fehniger TA., Ruggeri L., Velardi A., Caligiuri MA. Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood. 2002. 100:1935–47.

11). Phillips JH., Lanier LL. A model for the differentiation of human natural killer cells. Studies on the in vitro activation of Leu-11+ granular lymphocytes with a natural killer-sensitive tumor cell, K562. J Exp Med. 1985. 161:1464–82.

12). Warren HS., Skipsey LJ. Phenotypic analysis of a resting subpopulation of human peripheral blood NK cells: the FcR gamma III (CD16) molecule and NK cell differentiation. Immunology. 1991. 72:150–7.
13). Ishikawa E., Tsuboi K., Saijo K, et al. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004. 24:1861–71.
14). Ruggeri L., Capanni M., Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002. 295:2097–100.

15). Giebel S., Locatelli F., Lamparelli T, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003. 102:814–9.

16). Miller JS., Soignier Y., Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005. 105:3051–7.

17). Kogure T., Mantani N., Sakai S., Shimada Y., Tamura J., Terasawa K. Natural killer cytolytic activity is associated with the expression of killer cell immunoglobulin-like receptors on peripheral lymphocytes in human. Mediators Inflamm. 2003. 12:117–21.

18). Shin EC., Choi KS., Kim SJ., Shin JS. Modulation of the surface expression of CD158 killer cell Ig-like receptor by interleukin-2 and transforming growth factor-beta. Yonsei Med J. 2004. 45:510–4.
19). Vitale M., Bottino C., Sivori S, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998. 187:2065–72.

20). Castriconi R., Cantoni C., Della Chiesa M, et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci U S A. 2003. 100:4120–5.
Fig. 1
The change of CD158b expression on gated CD3- CD56+ NK cells in response to IL-2 alone and IL-2 plus K562 cell line. Representative data using PE-labeled CD 158b was shown. The increased expression was observed for CD 158b receptors, indicating that IL-2 alone (B) and IL2+K562 (C) induce up-regulation of CD158b compared to uncultured cells (A).

Fig. 2
Flow cytometric measurement of NK cytotoxicity using staining with FITC-congugated anti-CD45 and side scatter profiles to identify different cell populations and uptake of propidium iodide (PI) to detect cell death. Target cells and effectors (1:5) incubated for 4 hrs, are identified by region 1 (R1) and region 2 (R2) repectively. Percentages of dead cells in different regions are calculated from histograms showing PI uptake as measured by the intensity of fluorescence.
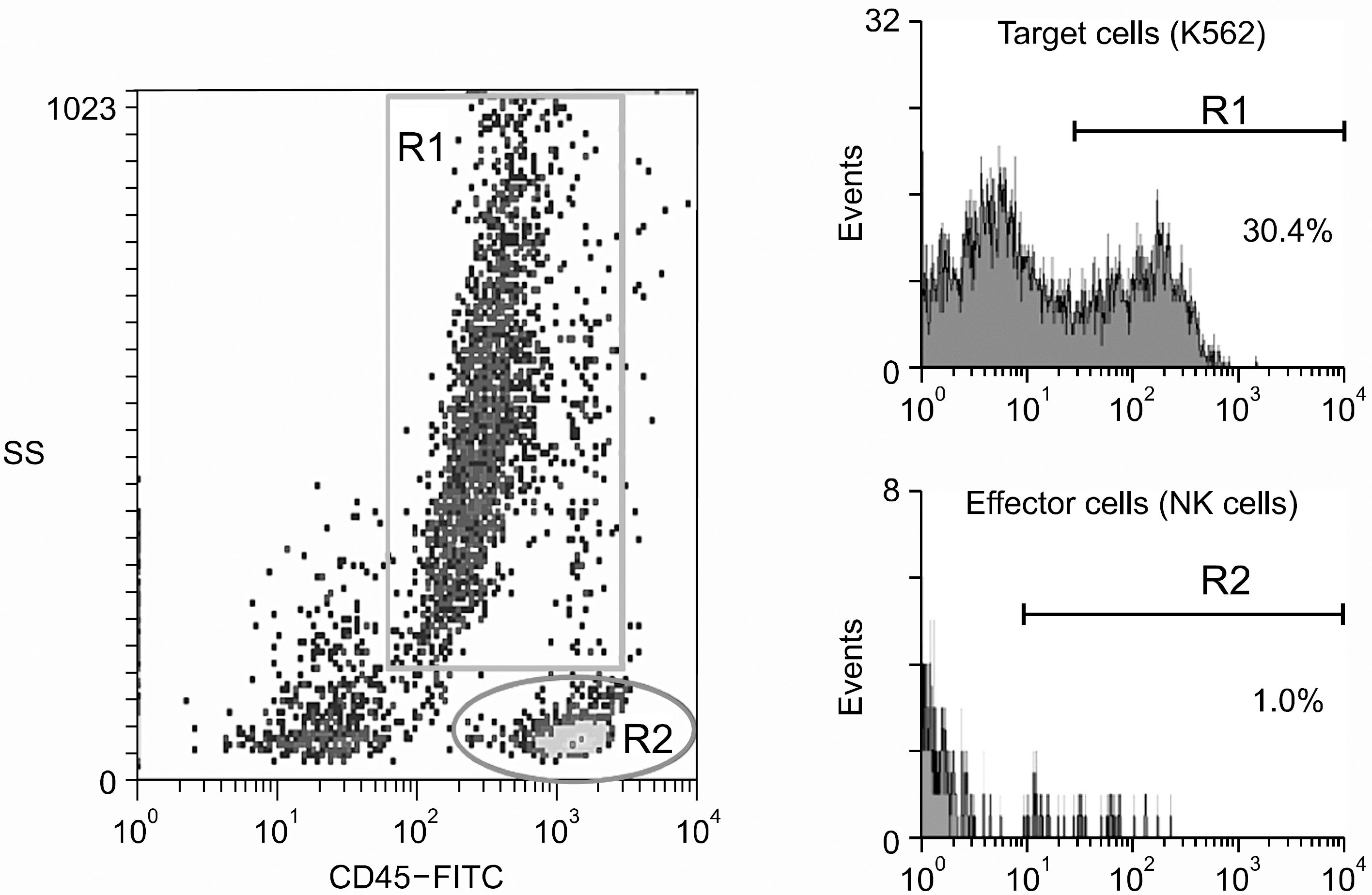
Table 1.
Median cell count, percentage of CD3-CD56+ cells, and fold expansion rate from peripheral blood mononuclear cells of seven donors. The cells were cultured in IL-2 (500U/mL) and irradiated K562 cell line as a feeder cell
Table 2.
The change of activating and inhibitory natural killer cell receptors when lymphocytes are treated with IL-2 alone or IL-2 with irradiated K562 cell line as feeder cell




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download