Abstract
Infectious mononucleosis caused by primary infection of Epstein-Barr virus (EBV) is a self-limiting lymphoproliferative disease, and shows concomitant clinical features such as pyrexia, anorexia, sore throat, cervical lymphadenopathy, liver dysfunction and hepatosplenomegaly. In rare cases, EBV establishes a latent infection in B lymphocytes and runs a chronic course and shows infectious mononucleosis-like symptoms repeatedly. This syndrome, named chronic active EBV infection, may trigger an autoimmune disease that mainly affectes the liver and red blood cells, and carries a very poor prognosis. The cardiovascular complications of chronic active EBV infection are very rare and may be associated with coronary arterial disease. This case describes a 5-year-old boy, who developed chronic active EBV infection and was diagnosed as having autoimmune hepatitis with a coronary aneurysm.
Go to : 
REFERENCES
1). Cho HS., Kim IS., Park HC., Ahn MJ., Lee YY., Park CK. A case of severe chronic active Epstein-Barr virus infection with T-cell lymphoproliferative disorder. Korean J Intern Med. 2004. 19:124–7.

3). Jones JF., Shurin S., Abramowsky C, et al. T-cell lymphomas containing Epstein-Barr viral DNA in patients with chronic Epstein-Barr virus infections. N Engl J Med. 1988. 318:733–41.

4). Kawa-Ha K., Ishihara S., Ninomiya T, et al. CD3-negative lymphoproliferative disease of granular lymphocytes containing Epstein-Barr viral DNA. J Clin Invest. 1989. 84:51–5.

5). Nakagawa A., Ito M., Iwaki T., Yatabe Y., Asai J., Hayashi K. Chronic active Epstein-Barr virus infection with giant coronary aneurysms. Am J Clin Pathol. 1996. 105:733–6.

6). Murakami K., Ohsawa M., Hu SX., Kanno H., Aozasa K., Nose M. Large-vessel arteritis associated with chronic active Epstein-Barr virus infection. Arthritis Rheum. 1998. 41:369–73.

7). Kikuta H., Sakiyama Y., Matsumoto S, et al. Detection of Epstein-Barr virus DNA in cardiac and aortic tissues from chronic, active Epstein-Barr virus infection associated with Kawasaki disease-like coronary artery aneurysms. J Pediatr. 1993. 123:90–2.

8). Hsiao CC. Epstein-Barr virus associated with immune thrombocytopenic purpura in childhood: a retrospective study. J Pediatr Child Health. 2000. 36:445–8.

9). Nickerson C., Luthra H., David C. Antigenic mimicry and autoimmune disease. Int Rev Immunol. 1991. 7:205–24.
10). Vento S., Guella L., Mirandola F, et al. Epstein-Barr virus as a trigger autoimmune hepatitis in susceptible individuals. Lancet. 1995. 346:608–9.
11). Kikuta H., Mizuno F., Osato T, et al. Kawasaki disease and unusual primary infection with Epstein-Barr virus. Pediatrics. 1984. 73:413–4.
12). Bernard O., Hadchouel M., Scotto J., Odievre M., Alagille D. Severe giant cell hepatitis with autoimmune hemolytic anemia in early childhood. J Pediatr. 1981. 99:704–11.

13). Melendez HV., Rela M., Baker AJ, et al. Liver trans-plant for giant cell hepatitis with autoimmune haemolytic anemia. Arch Dis Child. 1997. 77:249–51.
14). Taketani T., Kikuchi A., Inatomi J, et al. Chronic active Epstein-Barr virus infection (CAEBV) successfully treated with allogenic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2002. 29:531–3.
15). Fujii N., Takenaka K., Hiraki A, et al. Allogenic peripheral blood stem cell transplatation for the treatment of chronic Epstein-Barr virus infection. Bone Marrow Transplant. 2000. 26:805–8.
Go to : 
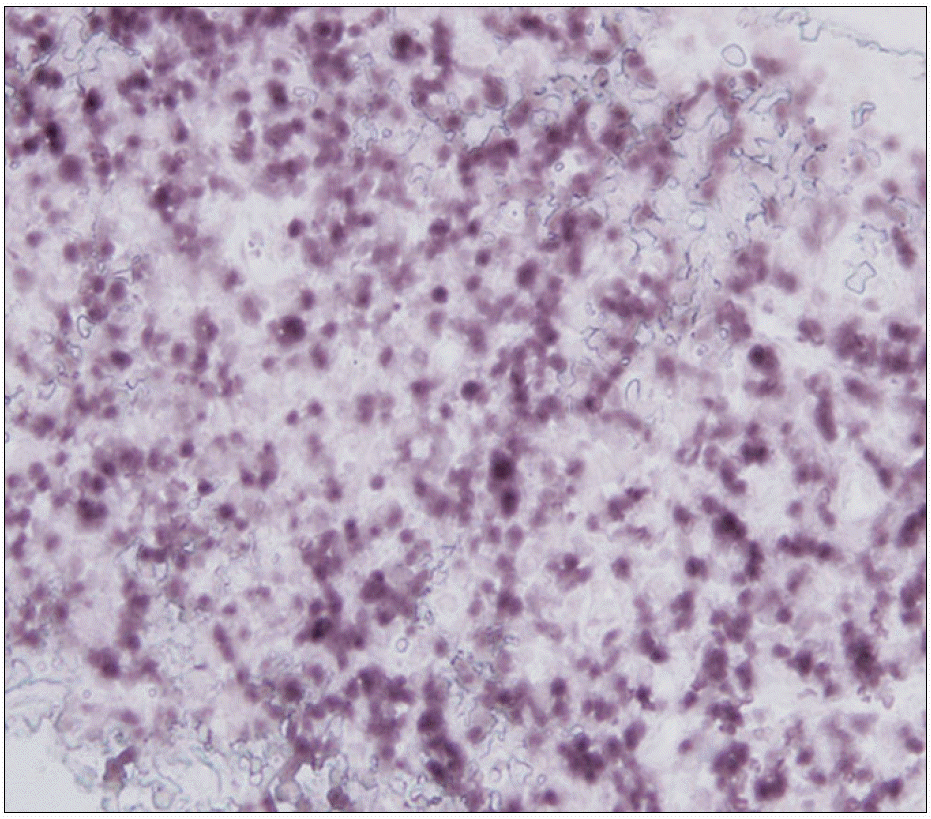 | Fig. 2EBV was observed in many lymphocytes by in situ hybridization in the cervical lymph node (dark stained cells) (immunochemicalhistostaining, ×400). |
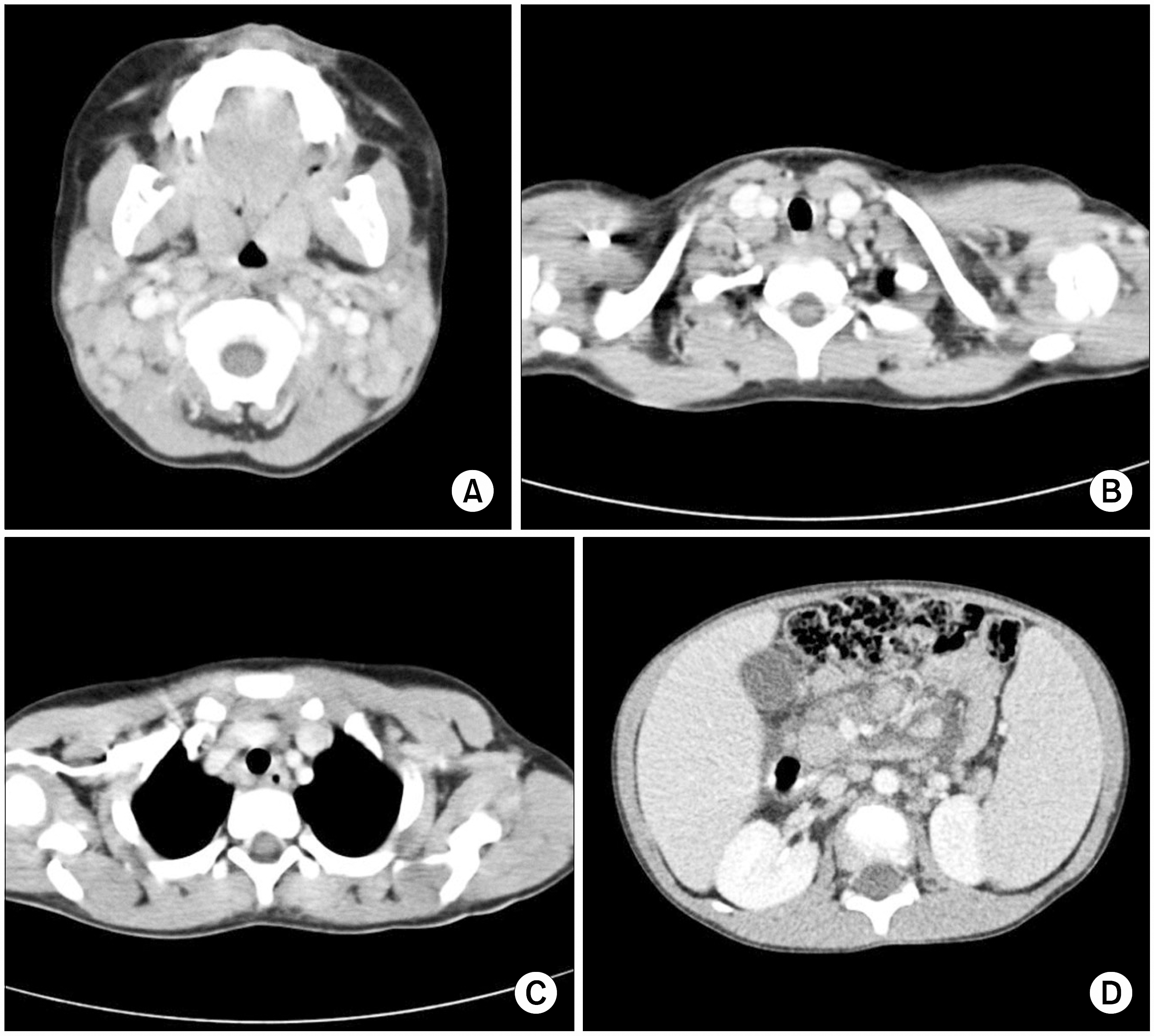 | Fig. 3Multiple conglomerated lymph nodes are found around both upper internal jugular, mid jugular, lower jugular, posterior triangular, submandibular and submental area (A), supraclavicular area (B), Rt. subcarina, paraesophageal area (C), abdominal aorta, inferior vena cava, celiac axis and mesenteric roots (D). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download