Abstract
Background:
Intravenous injection of mesenchymal and hematopoietic stem cells (MSCs, HSCs) has the disadvantages of low delivery rate to bone marrow and sequestration of cells in the lung and liver. This study was designed to determine whether there is a relationship between the administration route and dosage of stem cells and GVHD and survival.
Methods:
MSCs were retrieved from five subcultured C3H/10T1/2, cell lines from C3H/He mice. HSCs were transplanted by injecting 1×107 of bone marrow mononuclear cells and 5×106 of spleen cells from six to eight week old female C3H/He mice into six week old irradiated female BALB/c mice. The groups were divided into intravenous injection (IV) and intra-marrow (IM) injection groups. IV and IM+MSC groups consisted of mice transplanted with the same bone marrow mononuclear cells and SP, IV and IM groups, with the additional co-injection of 1×106 MSCs.
Go to : 
REFERENCES
1). Torok-Storb B., Holmberg L. Role of marrow micro-environment in engraftment and maintenance of allogeneic hematopoietic stem cells. Bone Marrow Transplant. 1994. 14:S71–3.
2). Fouillard L., Bensidhoum M., Bories D, et al. Engraftment of allogeneic mesenchymal stem cells in the bone marrow of a patient with severe idiopathic aplastic anemia improves stroma. Leukemia. 2003. 17:474–6.

3). Koc ON., Gerson SN., Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000. 18:307–16.
4). Noort WA., Kruisselbrink AB., in't Anker PS, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002. 30:870–8.

5). Tse WT., Pendleton JD., Beyer WM., Egalka MC., Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003. 75:389–97.

6). Gao J., Dennis JE., Muzic RF., Lundberg M., Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001. 169:12–20.

7). Zhang Y., Yasumizu R., Sugiura K, et al. Fate of allogeneic or syngeneic cells in intravenous or portal vein injection: possible explanation for the mechanism of tolerance induction by portal vein injection. Eur J Immunol. 1994. 24:1558–65.

8). Rombouts WJ., Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003. 17:160–70.

9). Kim JH., Auerbach JM., Rodriguez-Gomez JA, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002. 418:50–6.

10). Stamm C., Westphal B., Kleine HD, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003. 361:45–6.

11). Ikehara S. A novel strategy for allogeneic stem cell transplantation: perfusion method plus intra-bone marrow injection of stem cells. Exp Hematol. 2003. 31:1142–6.

12). Mazurier F., Doedens M., Gan OI., Dick JE. Rapid myeloerythroid repopulation after intrafemoral transplantation of NOD-SCID mice reveals a new class of human stem cells. Nat Med. 2003. 9:959–63.

13). Chung NG., Jeong DC., Park SJ, et al. Cotransplan-tation of marrow stromal cells may prevent lethal graft-versus-host disease in major histocompatibility complex mismatched murine hematopoietic stem cell transplantation. Int J Hematol. 2004. 80:370–6.

14). Jeong DC., Han CW., Jin JY, et al. Effectiveness of rotor off fraction in allogeneic murine bone marrow transplantation with complete disparity of major histocompatibility. Exp Hamatol. 1999. 27:1219–25.

15). Cooke KR., Kobzik L., Martin TR, et al. An experi mental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996. 88:3230–9.
16). Fridenshtein AIa. Stromal bone marrow cells and the hematopoietic microenvironment. Arkh Patol. 1982. 44:3–11.
17). Javazon EH., Beggs KJ., Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004. 32:414–25.

18). Krampera M., Glennie S., Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003. 101:3722–9.

19). Bartholomew A., Sturgeon C., Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002. 30:42–8.

20). Di Nicola M., Carlo-Stella C., Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002. 99:3838–43.

21). Meisel R., Zibert A., Laryea M., Gobel U., Daubener W., Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004. 103:4619–21.

22). Goker H., Haznedaroglu IC., Chao NJ. Acute graft-vs-host disease: pathobiology and management. Exp Hematol. 2001. 29:259–77.

23). Ferrara JL., Cooke KR., Pan L., Krenger W. The im-munopathophysiology of acute graft-versus-host-disease. Stem Cells. 1996. 14:473–89.

Go to : 
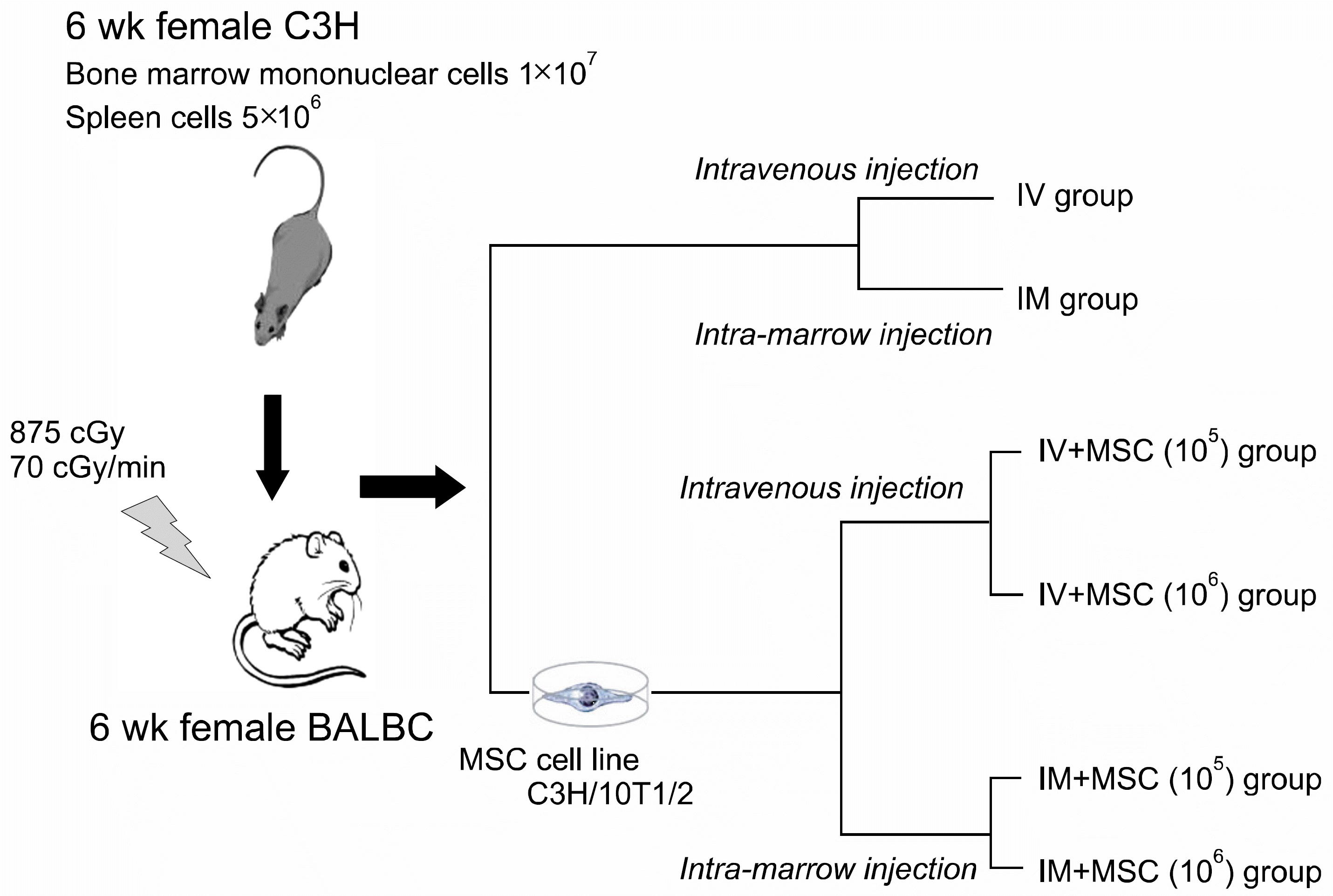 | Fig. 1The schematic diagram of MHC mismatched murine hematopoietic stem cell transplantation experimental model (MSC, mesenchymal stem cell; IV, intravenous injection; IM, intra-marrow injection). |
 | Fig. 2The severity and pattern of GVHD progression in the experimental group (BALB/c mouse, donor; C3H/He mouse, recipient) using GVHD scoring system of Cooke’s. There is no severe GVHD in all groups and no statistical significance (P>0.05). (IV group, 1×107 bone marrow mononuclear cells and 3×107 spleen cells intravenously co-injection; IV+MSC group, IV group+1×106 MSC intravenously co-injection; IM group, 1×107 bone marrow mononuclear cells and 3×107 spleen cells intra-marrow co-injection; IM+MSC group, IM group+1×106 MSC intra-marrow co-injection). MSC, mesenchymal stem cell. |
 | Fig. 3Skin and small intestine pathology in BALB/c mouse on 4 days and 8 days after allogenic hematopoietic stem cell transplantation (H&E stain). Skin and small intestine were harvested and prepared for microscopic analysis as described in materials and methods. Interstitial inflammation is observed involving epithelium, mucosae, vessels, parenchyma, and luminal structures on the post transplantation 8 day comparing to the 4th day. Predominantly mononuclear infiltrates and destructions of mucosae and epithelium are also observed. |
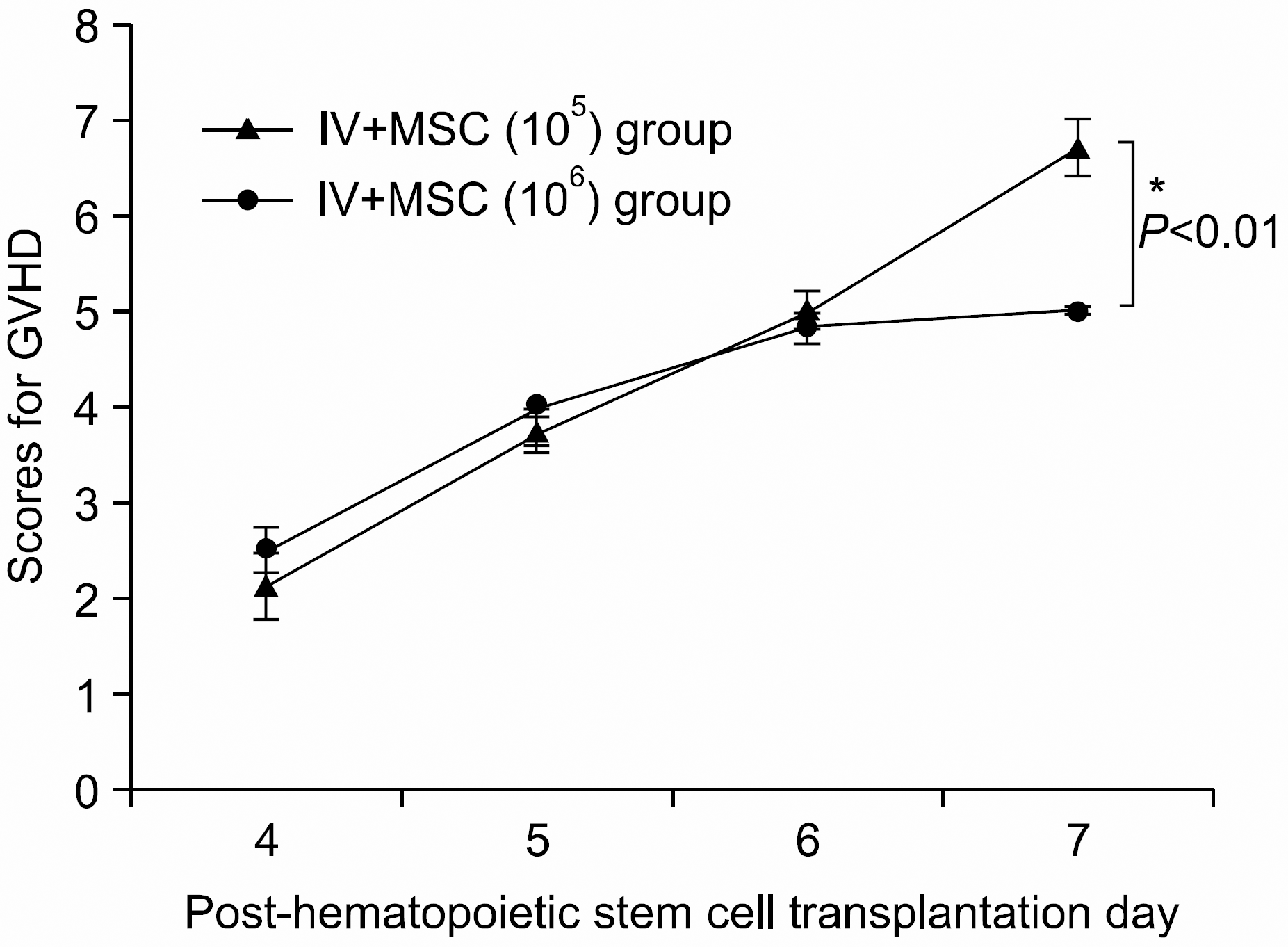 | Fig. 4The severity and pattern of GVHD progression in the intravenous mesenchymal stem cell co-injection hematopoietic stem cell transplantation experimental group. High dose MSC group shows a statistically significant lower GVHD scores from post-hematopoietic stem cell transplantation day 7 onwards compared with the other group (∗P<0.05). All data were shown mean±SD. (IV+MSC (105) group (n=7), 1×107 bone marrow mononuclear cells+5×10 6 spleen cells+1×105 MSC; IV+MSC (106) group (n=6), 1×107 bone marrow mononuclear cells+5×106 spleen cells+1×106 MSC). |
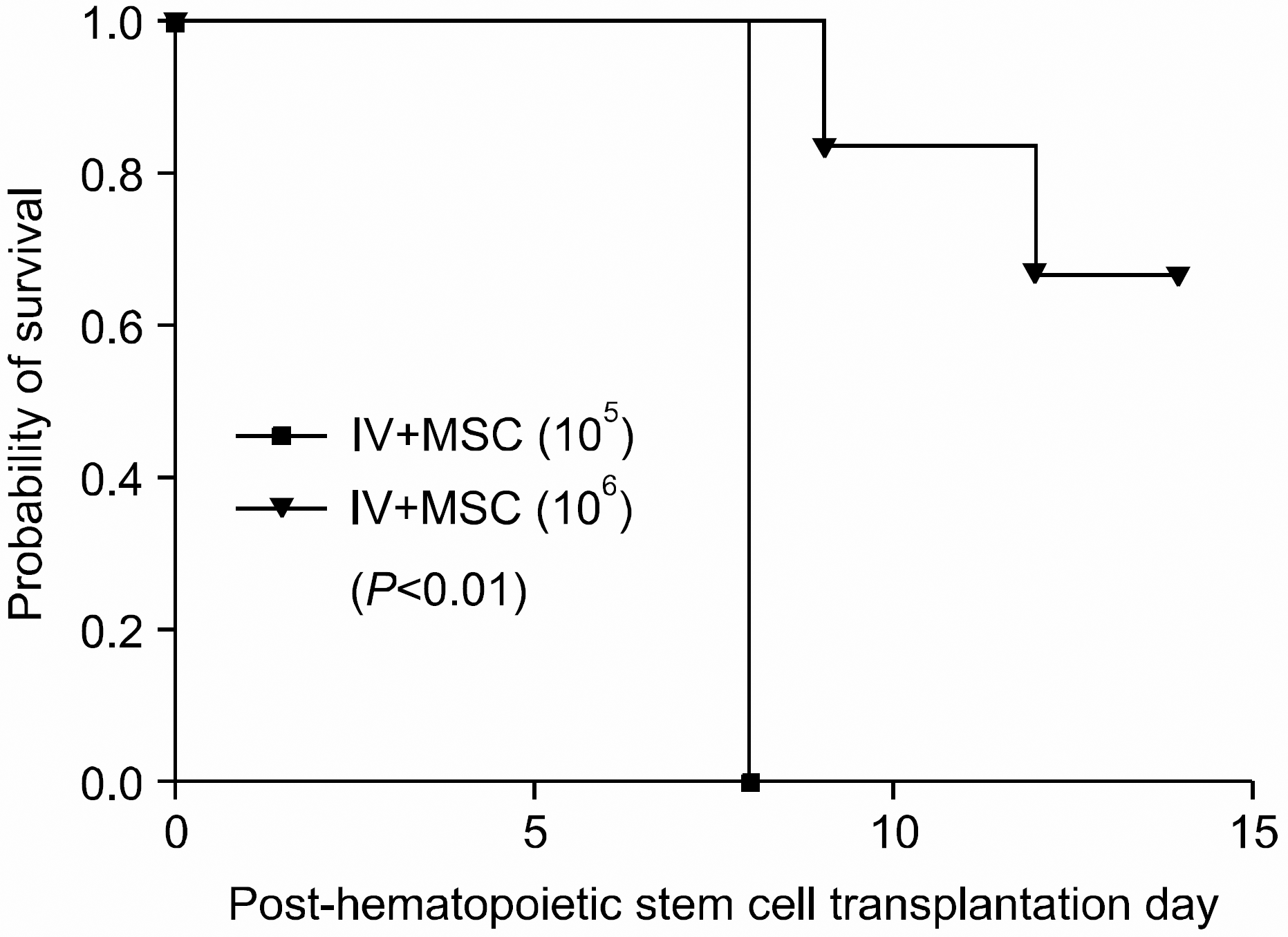 | Fig. 5Survival in BALB/c mice in intravenous mesenchymal stem cell co-injection hematopoietic stem cell transplantation experimental group. Whilst all mice in IV+MSC (105) group died in post-transplantation day 8, 4 mice in high dose MSC group survived until post-transplantation day 14, showing improved survival compared with the other group (P<0.01). (IV+MSC (105) group (n=7), 1×107 bone marrow mononuclear cells+5×106 spleen cells+ 1×105 MSC; IV+MSC (106) group (n=6), 1×107 bone marrow mononuclear cells+5×106 spleen cells+1×106 MSC). |
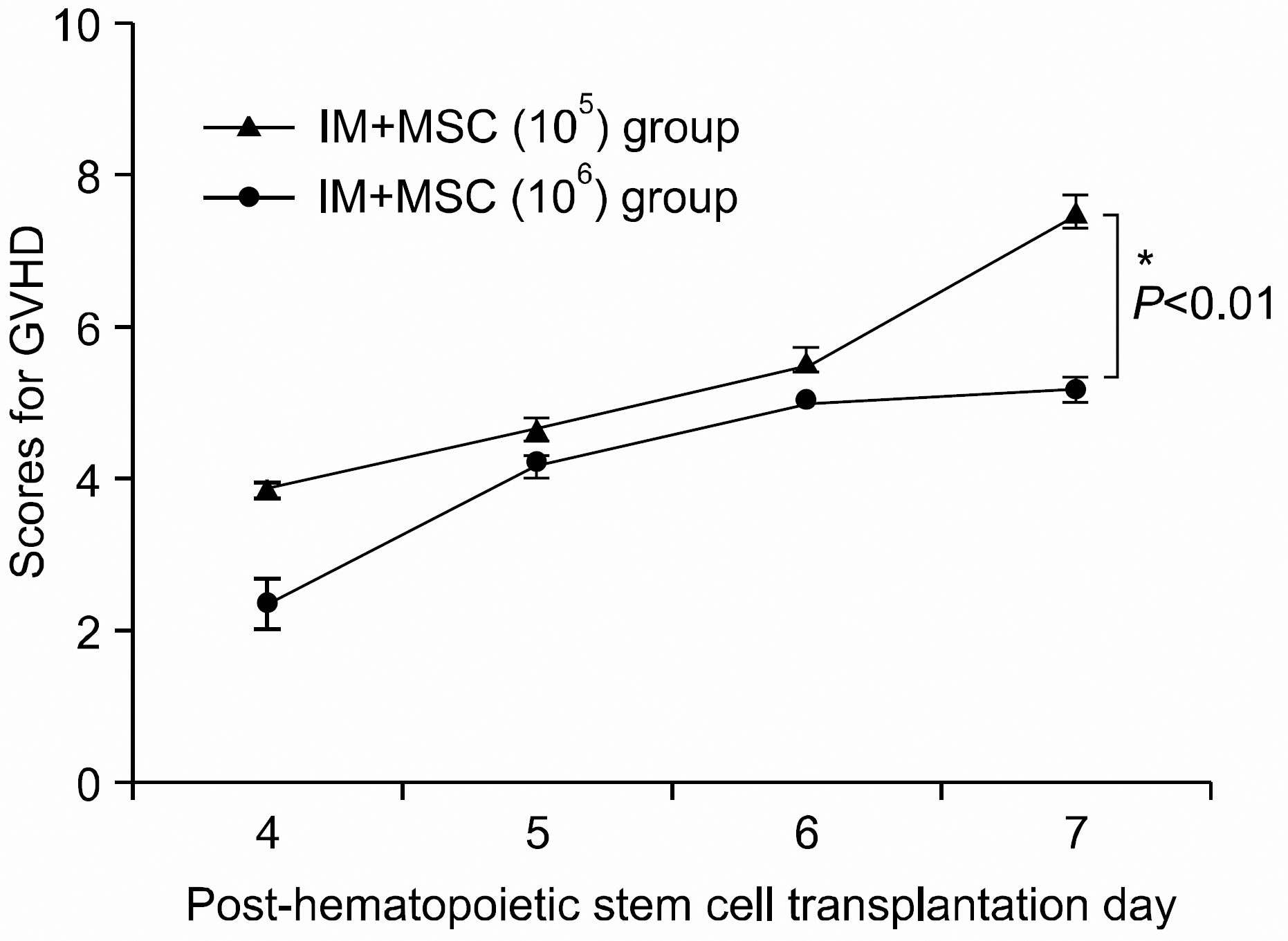 | Fig. 6The severity and pattern of GVHD progression in the intra-marrow mesenchymal stem cell co-injection hematopoietic stem cell transplantation experimental group. High dose MSC group shows a statistically significant lower GVHD scores (P<0.01) from post-hematopoietic stem cell transplantation Day 7 onwards compared with the other group (∗P<0.01). All data were shown mean±SD. (IM+ MSC (105) group (n=7), 1×107 bone marrow mononuclear cells+5×106 spleen cells+1×105 MSC; IM+MSC (106) group (n=6), 1×107 bone marrow mononuclear cells+5× 106 spleen cells+1×106 MSC). |
 | Fig. 7Survival in BALB/c mice in intra-marrow mesenchymal stem cell co-injection hematopoietic stem cell transplantation experimental group. Whilst all mice in IM group died in post-transplantation day 8, mouse in high dose MSC group survived until post-transplantation day 11, showing improved survival compared with the other group (P<0.01). (IM+MSC (105) group (n=7), 1×107 bone marrow mononuclear cells+5×106 spleen cells+1×105 MSC; IM+MSC (106) group (n=6), 1×107 bone marrow mononuclear cells+5×106 spleen cells+1×106 MSC). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download