Abstract
Background:
In multiple myeloma (MM), the idiotype (ID) determinant of the paraprotein has been used for immunotherapy using dendritic cells (DCs). However, ID-specific immune responses showed limited clinical responses after the Id vaccination. Therefore, an alternative approach using DCs pulsed with other tumor antigens is required.
Methods:
We investigated the possibility of immunotherapy for MM using myeloma cell line-specific cytotoxic T lymphocytes (CTLs), that were stimulated in vitro by monocyte-derived DCs pulsed with the myeloma cell line ysates. CD14+ cells isolated from the peripheral blood of HLA-A0201+ healthy donors were cultured in the presence of GM-CSF and IL-4. On day 6, the immature DCs were pulsed with the myeloma cell line lysates (IM-9: HLA0201+ and ARH-77: HLA0201+), and then maturation of DCs was induced by the addition of TNF-α for 2 days. CTL lines were generated by a 2 time stimulation with DCs to the autologous CD3+ T cells.
Results:
DCs pulsed with myeloma cell lysates showed the production of IL-12p70, but less than that of unpulsed DCs. CTLs lines stimulated with the DCs pulsing, for the myeloma cell line lysates, showed potent cytotoxic activities against autologous target cells, but not against HLA-A2- cell lines (RPMI-8226). Mature DCs pulsed with the myeloma cell line lysates showed a higher stimulatory capacity for autologous CTL when compared with mature non-pulsed DCs.
Go to : 
REFERENCES
3). Harrison SJ., Cook G. Immunotherapy in multiple myeloma—possibility or probability? Br J Haematol. 2005. 130:344–62.
4). Tricot G., Vesole DH., Jagannath S., Hilton J., Munshi N., Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood. 1996. 87:1196–8.

5). Perez-Simon JA., Martino R., Alegre A, et al. Chronic but not acute graft-versus-host disease improves outcome in multiple myeloma patients after non-myeloablative allogeneic transplantation. Br J Haematol. 2003. 121:104–8.
6). Bergenbrant S., Yi Q., Osterborg A, et al. Modulation of anti-idiotypic immune response by immunization with the autologous M-component protein in multiple myeloma patients. Br J Haematol. 1996. 92:840–6.

7). Osterborg A., Yi Q., Henriksson L, et al. Idiotype immunization combined with granulocyte-macrophage colony-stimulating factor in myeloma patients induced type I, major histocompatibility complex-restricted, CD8- and CD4-specific T-cell responses. Blood. 1998. 91:2459–66.
8). Wen YJ., Ling M., Bailey-Wood R., Lim SH. Idiotypic protein-pulsed adherent peripheral blood mononuclear cell-derived dendritic cells prime immune system in multiple myeloma. Clin Cancer Res. 1998. 4:957–62.
9). Lim SH., Bailey-Wood R. Idiotypic protein-pulsed dendritic cell vaccination in multiple myeloma. Int J Cancer. 1999. 83:215–22.

10). Reichardt VL., Okada CY., Liso A, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma—a feasibility study. Blood. 1999. 93:2411–9.

11). Lim SH., Bailey-Wood R. Idiotypic protein-pulsed dendritic cell vaccination in multiple myeloma. Int J Cancer. 1999. 83:215–22.

12). Osterborg A., Yi Q., Henriksson L, et al. Idiotype immunization combined with granulocyte-macrophage colony-stimulating factor in myeloma patients induced type I, major histocompatibility complex-restricted, CD8- and CD4-specific T-cell responses. Blood. 1998. 91:2459–66.
13). Wen YJ., Min R., Tricot G., Barlogie B., Yi Q. Tumor lysate-specific cytotoxic T lymphocytes in multiple myeloma: promising effector cells for immunotherapy. Blood. 2002. 99:3280–5.

14). Milazzo C., Reichardt VL., Muller MR., Grunebach F., Brossart P. Induction of myeloma-specific cytotoxic T cells using dendritic cells transfected with tumor-derived RNA. Blood. 2003. 101:977–82.

15). Qian J., Wang S., Yang J, et al. Targeting heat shock proteins for immunotherapy in multiple myeloma: generation of myeloma-specific CTLs using dendritic cells pulsed with tumor-derived gp96. Clin Cancer Res. 2005. 11:8808–15.

16). Gong J., Koido S., Chen D, et al. Immunization against murine multiple myeloma with fusions of dendritic and plasmacytoma cells is potentiated by interleukin 12. Blood. 2002. 99:2512–7.

17). Raje N., Hideshima T., Davies FE, et al. Tumour cell/dendritic cell fusions as a vaccination strategy for multiple myeloma. Br J Haematol. 2004. 125:343–52.

18). Hao S., Bi X., Xu S, et al. Enhanced antitumor immunity derived from a novel vaccine of fusion hybrid between dendritic and engineered myeloma cells. Exp Oncol. 2004. 26:300–6.
19). Vasir B., Borges V., Wu Z, et al. Fusion of dendritic cells with multiple myeloma cells results in maturation and enhanced antigen presentation. Br J Haematol. 2005. 129:687–700.

20). Lee JJ., Takei M., Hori S, et al. The role of PGE(2) in the differentiation of dendritic cells: how do dendritic cells influence T-cell polarization and chemokine receptor expression? Stem Cells. 2002. 20:448–59.
21). Lee JJ., Nam CE., Nam JH, et al. Generation of cytotoxic donor CD8+ T cells against relapsing leukemic cells following allogeneic transplantation by stimulation with leukemic cell- or leukemic lysate pulsed donor cell-derived dendritic cells. Leuk Res. 2004. 28:517–24.

22). Lee JJ., Kook H., Park MS, et al. Immunotherapy using autologous monocyte-derived dendritic cells pulsed with leukemic cell lysates for acute myeloid leukemia relapse after autologous peripheral blood stem cell transplantation. J Clin Apher. 2004. 19:66–70.

23). Lee JJ., Choi BH., Nam JH, et al. The generation of leukemic dendritic cells from acute myeloid leukemia cells is potentiated by the addition of CD40L at the terminal maturation stage. J Clin Apher. 2004. 19:130–6.

24). Fiore F., Nuschak B., Peola S, et al. Exposure to myeloma cell lysates affects the immune competence of dendritic cells and favors the induction of Tr1-like regulatory T cells. Eur J Immunol. 2005. 35:1155–63.
Go to : 
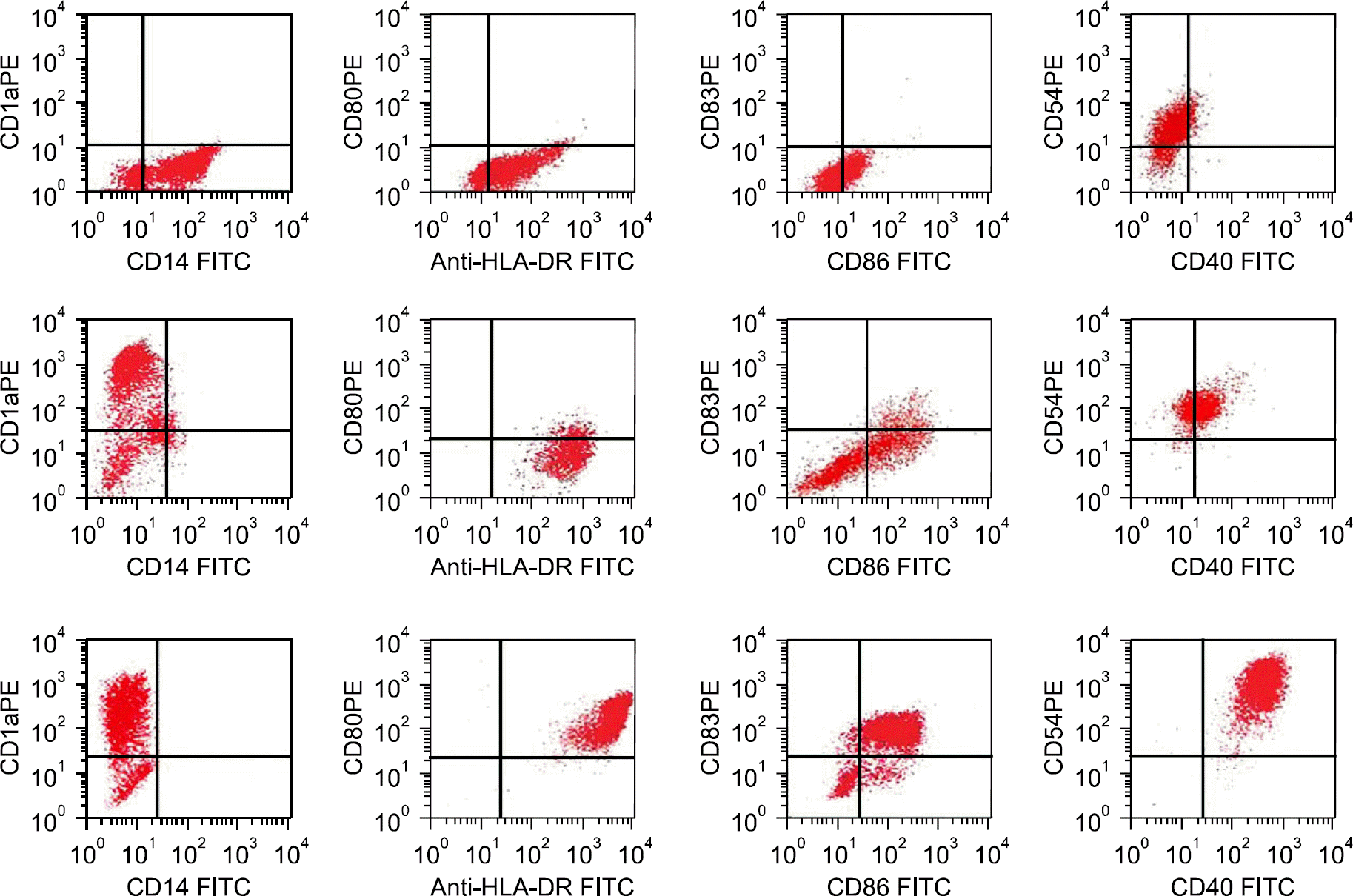 | Fig. 1Phenotypic analysis of monocytes (top panel), immature dendritic cells (DCs) (middle panel), or mature DCs (bottom panel). CD14+ cells isolated from the MNCs of normal donor by MACS were cultured with a combination of GM-CSF and IL-4. On day 6, the immature DCs (middle panel) were pulsed with tumor antigens, and then maturation was induced by the addition of TNF-α (bottom panel). |
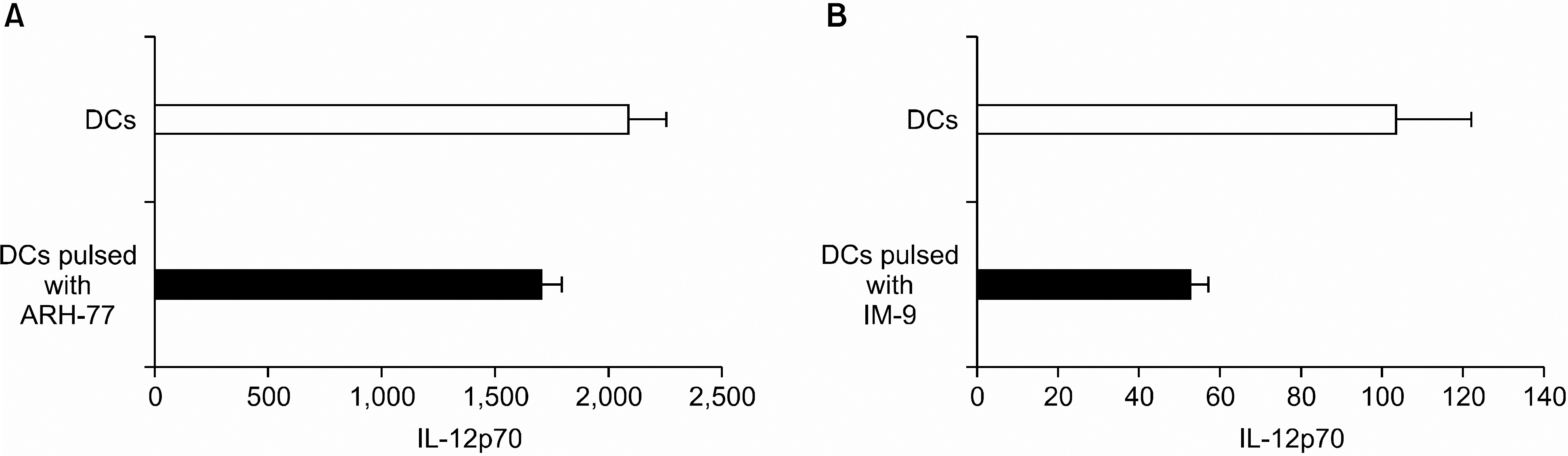 | Fig. 2Production of IL-12 by DCs pulsed with or without myeloma cell line lysates (A: ARH-77, B: IM-9) after the stimulation with CD40L-transfected J558 cells for 24 hr. IL-12p70 concentrations in 24 hr supernatants were determined by ELISA. Results, expressed as mean±SD of duplicate culture, are from one representative experiment of two. |
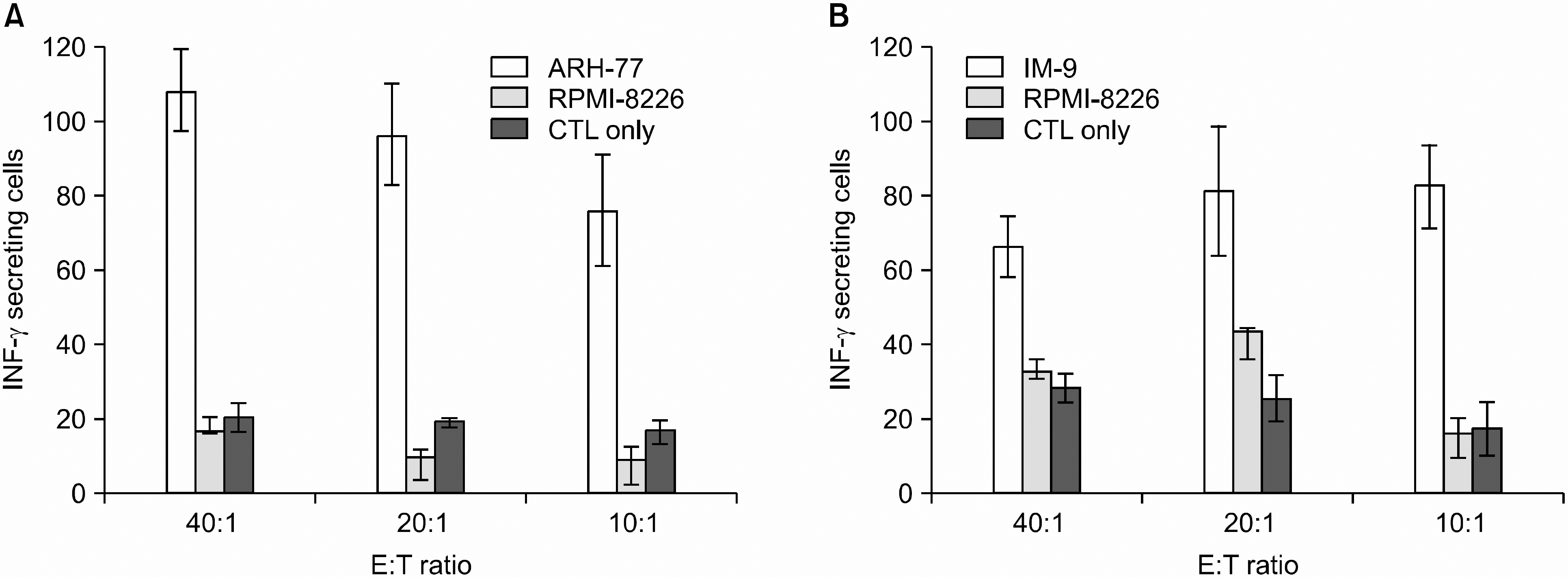 | Fig. 3ELISPOT data showing the mean number of spots and standard deviation per 1~4×105 T cells. Comparison of IFN-γ secreting cells of CTL generated from stimulation with DCs pulsed with ARH-77 lysates (A) or IM-9 lysates (B) against autologous myeloma cell line lysates or RPMI-8226. The data are shown as the mean IFN-γ secreting cell (±SD) of duplicate culture from one representative experiment of two. |
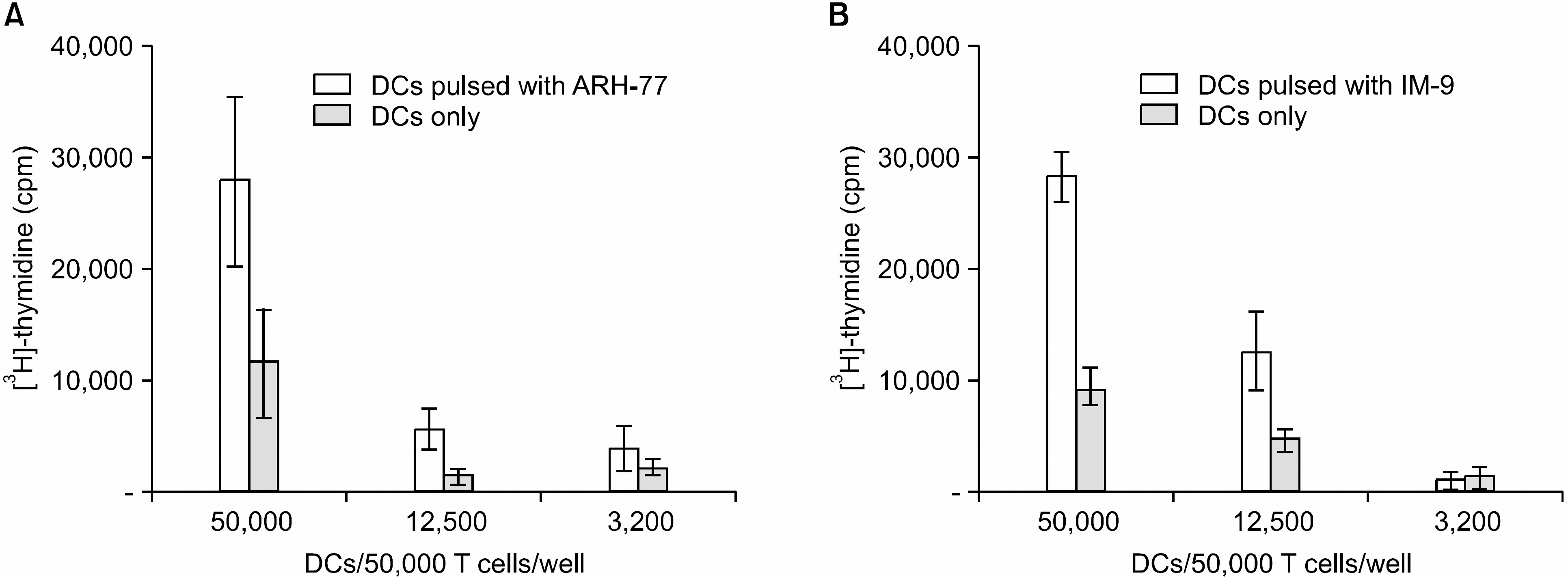 | Fig. 4T cell-stimulatory capacity of DCs with or without myeloma cell line lysates (ARH-77: A, IM-9: B) for CTLs that were generated from stimulation with DCs with myeloma cell line lysates. The data are shown as the mean cpm (±SD) of triplicate cultures from one representative experiment of two. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download