Abstract
Background:
We have determined the effects of human telomerase RNA inhibiton using siRNA in tumor cells and human embryonic and mesenchymal stem cells.
Methods:
We selected the sequences against the predicted loop; these sequneces were comprised of nucleotides from 76 to 94 residues and from 143 to 163 residues as the target sequences, and we cloned these sequences into pU6sh75 and pU6sh143 cells. Three different kinds of cell lines were used: HeLa, SNUhES3, and human mesenchymal stem cells. The degree of inhibition of telomerase activity was assessed by TRAP assay and RT-PCR.
Results:
The telomerase activity of the HeLa and SNUhES3 cells were 135.3±14.5 and 109.0±18.2; these cells showed higher activity than human mesenchymal stem cells and Wi38 cells (46.3±5.0 and 26.0±12.0), which were control cells. When each of the types of cells was treated with siRNA-hTR, the transfection efficiency of pU6sh75 for the HeLa, SNUhES3, and human mesenchymal stem cells was 91.0±8.4%, 83.3±16.0% and 81.9±12.3%, respectively. In the case of pU6sh143, its transfection efficiency was similar to pU6sh75; the HeLa, SNUhES3 and human mesenchymal stem cells tranfection efficiency was 90.1±9.0%, 79.9±18.2% and 79.4±15.1%, respectively. After two days of transfection, the level of telomerase activity in the pU6sh75 transfected cells decreased to 64.3±10.1% and 56.0±11.0% in the HeLa and SNUhES3 cells, respectively. When the cells were transfected with pU6sh143, the telomerase activity also decreased in the HeLa and SNUhES3 cells (71.3±9.1% and 61.6±8.3%, respectively). However, the difference of telomerase activity was not significant in the human mesenchymal stem cells: 43.0±7.2% with pU6sh75 and 46.0±9.0% with pU6sh143.
Go to : 
REFERENCES
1). Nakamura M., Masutomi K., Kyo S, et al. Efficient inhibition of human telomerase reverse transcriptase expression by RNA interference sensitizes cancer cells to ionizing radiation and chemotherapy. Hum Gene Ther. 2005. 16:859–68.

2). Blasco MA., Hahn WC. Evolving views of telomerase and cancer. Trends Cell Biol. 2003. 13:289–94.

3). Burns JS., Abdallah BM., Guldberg P., Rygaard J., Schroder HD., Kassem M. Tumorigenic heterogeneity in cancer stem cells evolved from long-term cultures of telomerase-immortalized human mesenchymal stem cells. Cancer Res. 2005. 65:3126–35.

4). Armstrong L., Saretzki G., Peters H, et al. Overex-pression of telomerase confers growth advantage, stress resistance, and enhanced differentiation of ESCs toward the hematopoietic lineage. Stem Cells. 2005. 23:516–29.

5). Feng J., Funk WD., Wang SS, et al. The RNA component of human telomerase. Science. 1995. 269:1236–41.

6). Liu L., DiGirolamo CM., Navarro PA., Blasco MA., Keefe DL. Telomerase deficiency impairs differentiation of mesenchymal stem cells. Exp Cell Res. 2004. 294:1–8.

7). Kondo Y., Koga S., Komata T., Kondo S. Treatment of prostate cancer in vitro and in vivo with 2-5A-anti-telomerase RNA component. Oncogene. 2000. 19:2205–11.

8). Komata T., Kondo Y., Koga S., Ko SC., Chung LW., Kondo S. Combination therapy of malignant glioma cells with 2-5A-antisense telomerase RNA and recombinant adenovirus p53. Gene Ther. 2000. 7:2071–9.

9). Elbashir SM., Harborth J., Weber K., Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002. 26:199–213.

10). Kondo S., Kondo Y., Li G., Silverman RH., Cowell JK. Targeted therapy of human malignant glioma in a mouse model by 2-5A antisense directed against telomerase RNA. Oncogene. 1998. 16:3323–30.

11). Ito H., Kyo S., Kanaya T., Takakura M., Inoue M., Namiki M. Expression of human telomerase subunits and correlation with telomerase activity in urothelial cancer. Clin Cancer Res. 1998. 4:1603–8.
12). Kosciolek BA., Kalantidis K., Tabler M., Rowley PT. Inhibition of telomerase activity in human cancer cells by RNA interference. Mol Cancer Ther. 2003. 2:209–16.
13). Shen C., Buck AK., Liu X., Winkler M., Reske SN. Gene silencing by adenovirus-delivered siRNA. FEBS Lett. 2003. 539:111–4.

Go to : 
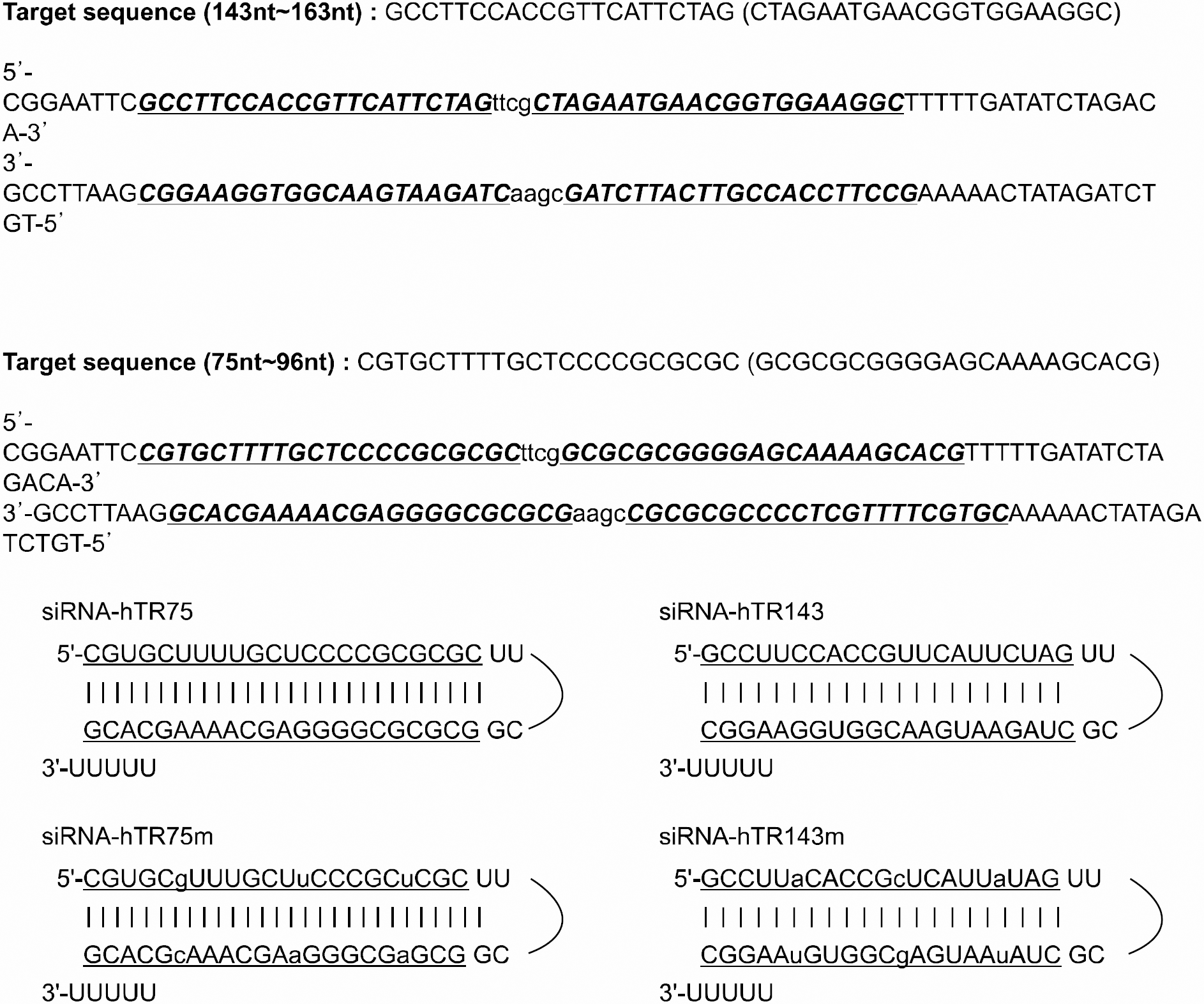 | Fig. 1Sequences and predicted secondary structure of hairpin RNAs derived from the U6 promoter-driven expression constructs. Mutant versions of each siRNA with 3 points mutation at the sequences of the target were also shown. |
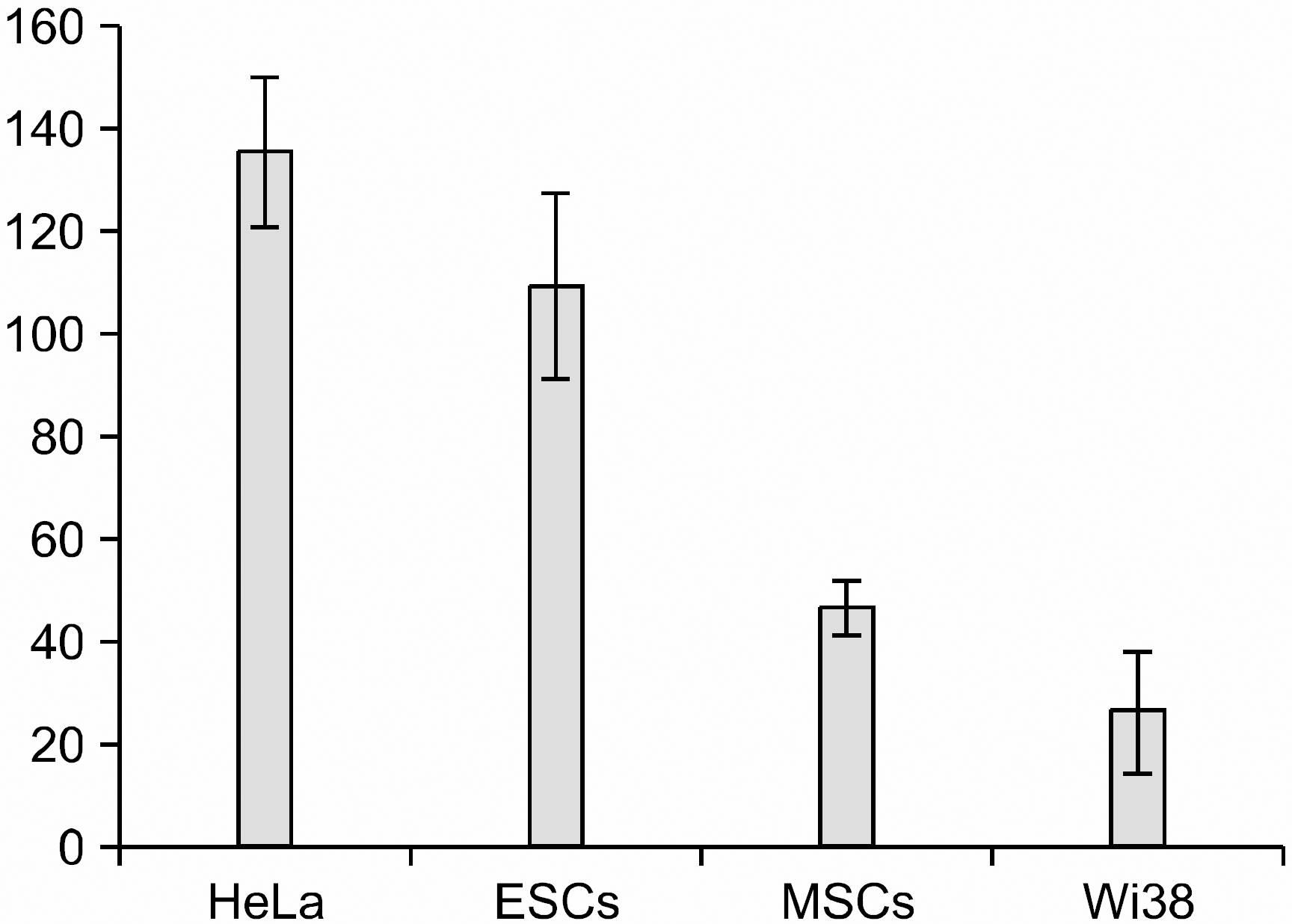 | Fig. 2Telomerase activity in experimental cell lines. The telomerase activity was assayed by a telomere repeat amplification protocol (TRAP) assay using 2,000 cells. The telomerase activity of the U293 cell line was considered as 100, with the telomerase activity of each cell line normalized to this. The means from at least three independent experiments are shown. |
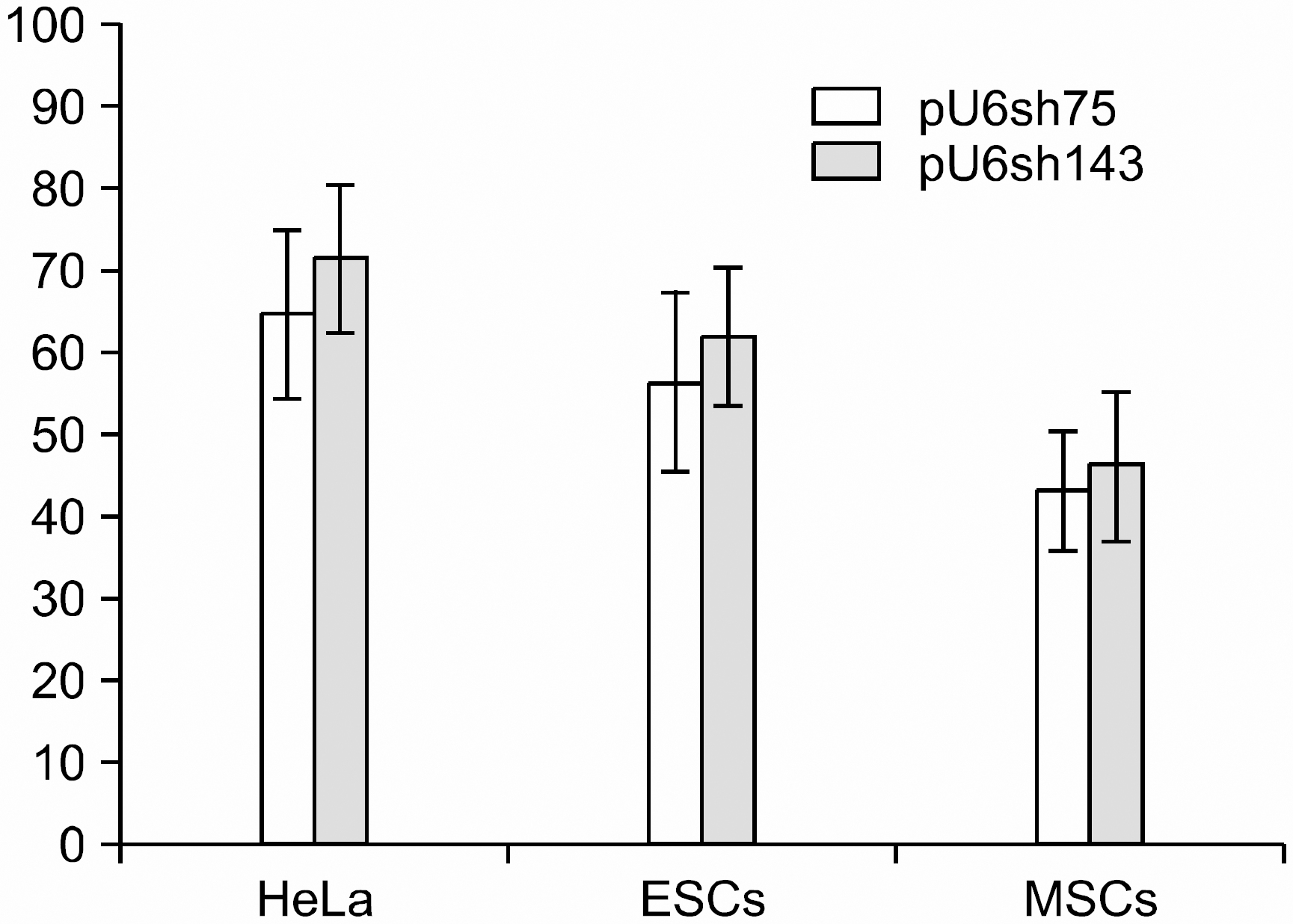 | Fig. 3Inhibition of Telomerase activity by siRNA. The effects of siRNAs are expressed as the percentage of the telomerase activity compared with mutant versions (hTR75 m, hTR143m). The means from at least three independent experiments are shown. |
 | Fig. 4Inhibition of mRNA expression of hTR. In the siRNA-hTR143 transfected ES, mRNA expression of hTR was decreased compared to control ES and siRNA-hTR143m transfected ES. M, marker for molecular weight; lane 1 and 4, control ES; lane 2 and 5, siRNA hTR143m transfected ES; lane 3 and 6, siRNA hTR143 transfected ES. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download