Abstract
Background:
Background Telomerase activation and human telomerase RNA (hTR) expression are known to be related to the preservation of the “stemness” of stem cells. In this study, we have inhibited the expression of hTR to find the relationship between the telomerase activity and differentiation of normal hematopoietic stem cells.
Methods:
We used cord blood collected from 10 full term pregnant women. We classified the CD34+ hematopoietic stem cells from the same donor into three groups: the Ad-OA group was treated with the recombinant adenoviral (Ad) vector Ad-OA using telomerase antisense, the Ad-M6 group was treated with a mutant version of the Ad-OA without telomerase antisense, and a control group without any treatment.
Results:
The mean number of colony-forming cells (CFCs) were 110±38 for the Ad-OA groups, 540±56 for the Ad-M6 groups, and 650±72 for the control groups. Thus, CFCs in the Ad-OA group were lower than in the Ad-M6 group (P<0.01). The myeloid portion of the CFCs in the Ad-OA group was higher than the Ad-M6 and control groups (P<0.01). The Ad-OA group showed a higher percentage of granulocytes suggesting more of a tendency for myeloid differentiation than the Ad-M6 and control groups (P<0.01). We found that the suppression of telomerase activity by the antisense telomerase adenovirus induced the differentiation of hematopoietic stem cells confirmed by differential cell count and cytochemical staining.
Go to : 
REFERENCES
1). Feng J., Funk WD., Wang SS, et al. The RNA component of human telomerase. Science. 1995. 269:1236–41.

2). Sharma HW., Sokoloski JA., Perez JR, et al. Differentiation of immortal cells inhibits telomerase activity. Proc Natl Acad Sci. 1995. 92:12343–6.

3). Kraveka JM., Li L., Bielawski J., Obeid LM., Ogretmen B. Involvement of endogenous ceramide in the inhi bition of telomerase activity and induction of morphologic differentiation in response to all-trans-retinoic acid in human neuroblastoma cells. Arch Biochem Biophys. 2003. 419:110–9.
4). Cheng AJ., Liao SK., Chow SE., Chen JK., Wang TC. Differential inhibition of telomerase activity during induction of differentiation in hematopoietic, melanoma, and glioma cells in culture. Biochem Biophys Res Commun. 1997. 237:438–44.

5). Liu L., Berletch JB., Green JG., Pate MS., Andrews LG., Tollefsbol TO. Telomerase inhibition by retinoids precedes cytodifferentiation of leukemia cells and may contribute to terminal differentiation. Mol Cancer Ther. 2004. 8:1003–9.
6). Zimmermann S., Glaser S., Ketteler R., Waller CF., Klingmuller U., Martens UM. Effects of telomerase modulation in human hematopoietic progenitor cells. Stem Cells. 2004. 22:741–9.

7). Song JS., Kim HP., Yoon WS, et al. Adenovirus-mediated suicide gene therapy using the human telomerase catalytic subunit (hTERT) gene promoter induced apoptosis of ovarian cancer cell line. Biosci Biotechnol Biochem. 2003. 67:2344–50.

8). Graham FL., Van Der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Viology. 1973. 52:456–67.

9). Liu T., Hu B., Chung MJ., Ullenbruch M., Jin H., Phan SH. Telomerase regulation of myofibroblast differentiation. Am J Respir Cell Mol Biol. 2006. 34:625–33.

10). Liu L., DiGirolamo CM., Navarro PA., Blasco MA., Keefe DL. Telomerase deficiency impairs differentiation of mesenchymal stem cells. Exp Cell Res. 2004. 10: 294:1–8.

11). Foster LJ., Zeemann PA., Li C., Mann M., Jensen ON., Kassem M. Differential expression profiling of membrane proteins by quantitative proteomics in a human mesenchymal stem cell line undergoing osteoblast differentiation. Stem Cells. 2005. 23:1367–7.

12). Armstrong L., Saretzki G., Peters H, et al. Overexpre-ssion of telomerase confers growth advantage, stress resistance, and enhanced differentiation of ESCs toward the hematopoietic lineage. Stem Cells. 2005. 23:516–29.

13). Lee MK., Hande MP., Sabapathy K. Ectopic mTERT expression in mouse embryonic stem cells does not affect differentiation but confers resistance to differentiation-and stress-induced p53-dependent apoptosis. J Cell Sci. 2005. 15:819–29.
Go to : 
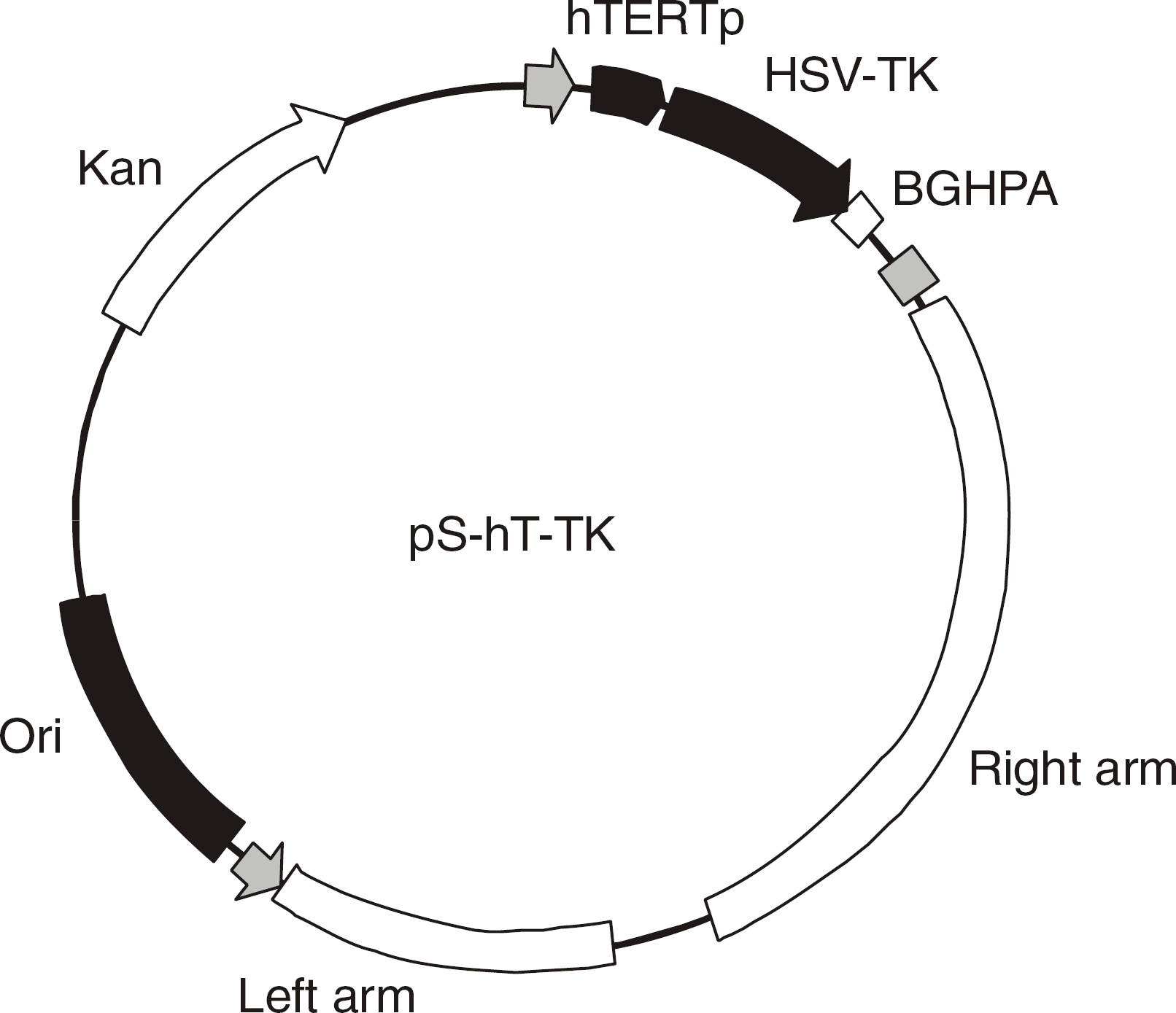 | Fig. 1Structure of recombinant adenoviral vector with deficient E1a/E1b. It contains residues 94 and 76 of the telomerase template sequence driven by the cytomegalovirus immediate promoter, inserted into the E1 region. |
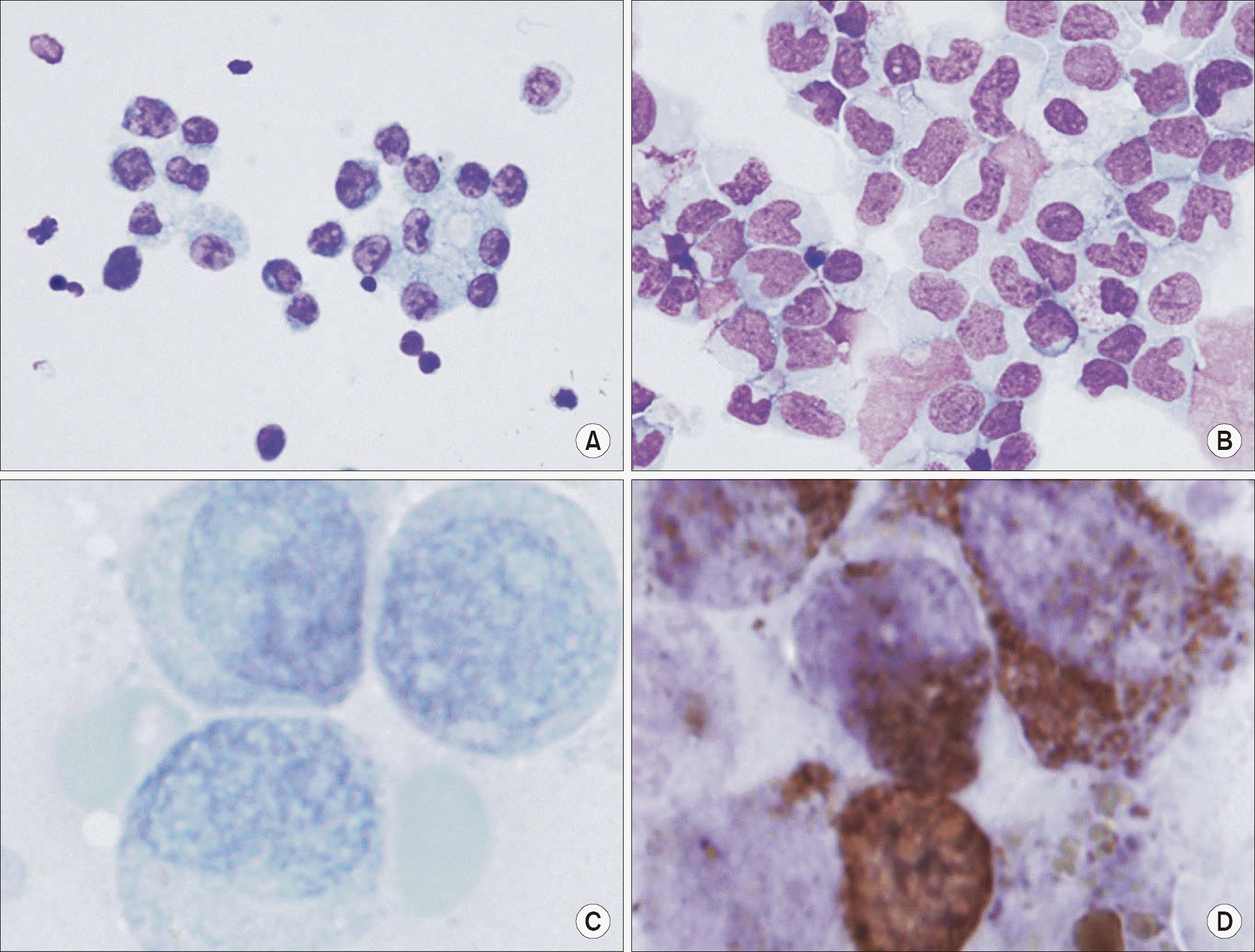 | Fig. 2Cell morphology in Ad-OA and Ad-M6 groups at day 7 of long-term culture with CD34+ cells. Top. Blood cell morphology with Wright Stain (×400): significantly higher percentage of granulocytes in the Ad-OA group compared to (B) those in the Ad-M6 group (A) this suggests a differentiation induction effect of telomerase antisense. Bottom. Granulocyte morphology of the myeloperoxidase stain (×1,000): There is a more dense myelopeoxidase stain pattern in the Ad-OA group when compared to (D) that in the Ad-M6 group (C) this suggests a maturation induction effect of telomerase antisense. |
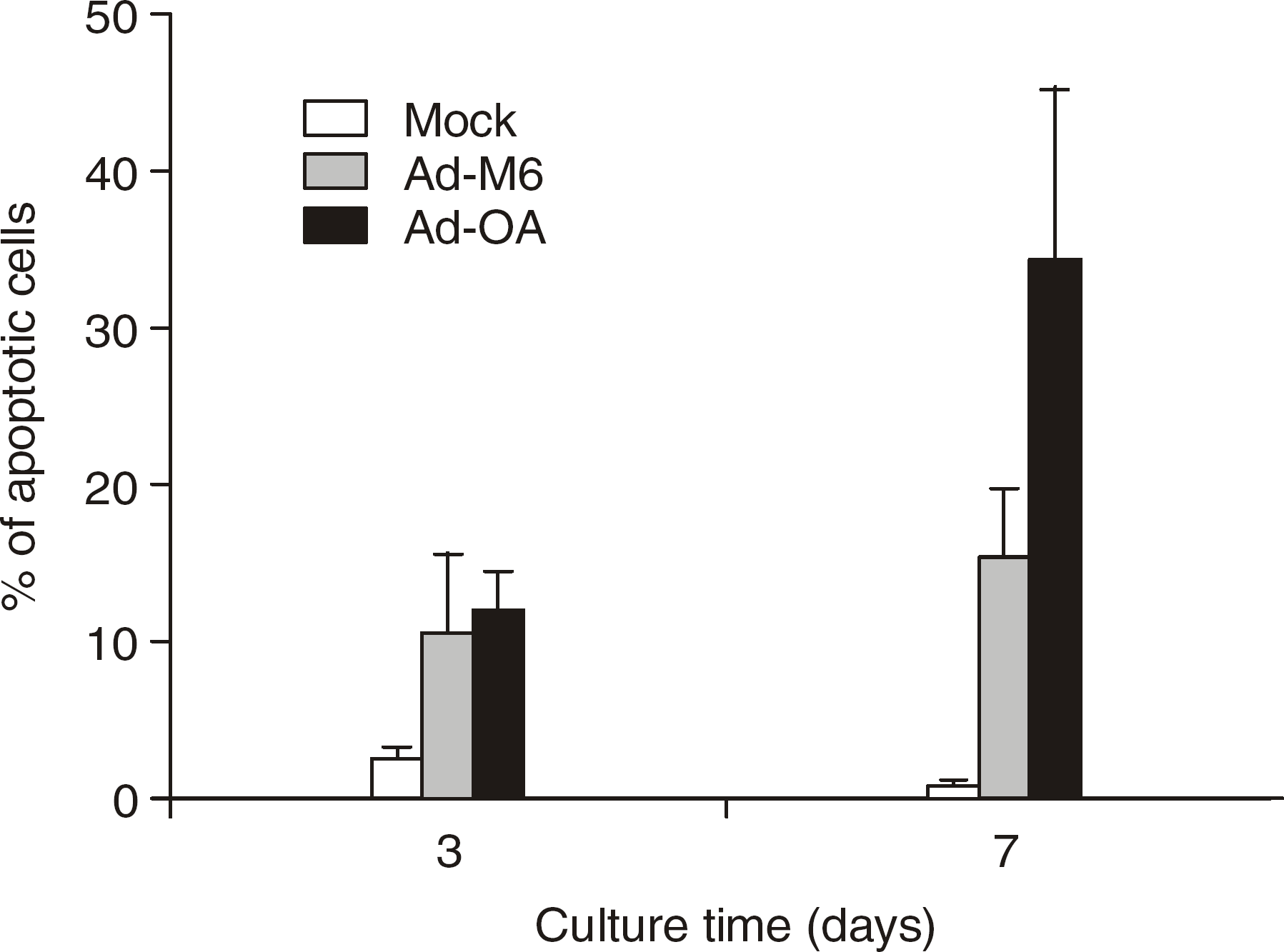 | Fig. 3The degree of apoptosis in CD34+ cells of Ad-OA, Ad-M6, and control Mock groups. The data represent mean values and standard deviation (S.D.) of three independent experiments. |
Table 1.
Differential cell counts of Ad-OA, Ad-M6, and control group a3 and 7 day after long-term culture with CD34+ cells
| Day 3 (%, mean±SEM) | Day 7 (%, mean±SEM) | |||||
|---|---|---|---|---|---|---|
| Ad-OA | Ad-M6 | Control | Ad-OA | Ad-M6 | Control | |
| Blast | 21±6 | 47±12 | 68±8 | 2±1 | 18±9 | 20±6 |
| Lymphocyte | 17±7 | 16±7 | 18±7 | 8±3 | 5±2 | 16±11 |
| Granulocyte | 45±5∗ | 12±6 | 7±3 | 33±12∗ | 9±3 | 16±5 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download