Abstract
Background
An angioimmunoblastic T-cell lymphoma (AITL) is a rare subtype of lymphoma, accounting for only 1 to 2% of studies on non-Hodgkin's lymphomas. Because of the rarity of this disease, most studies have been small, including cases of various T-cell Non-Hodgkin's Lymphoma (T-NHL). Those patients diagnosed as AITL, during the last 8 years at a single institution (Seoul National University Hospital), were retrospectively analyzed to determine the clinical features and treatment outcomes of AITL.
Methods
All 24 of the patients diagnosed with AITL between February 1995 and February 2003 were included in this retrospective review.
Results
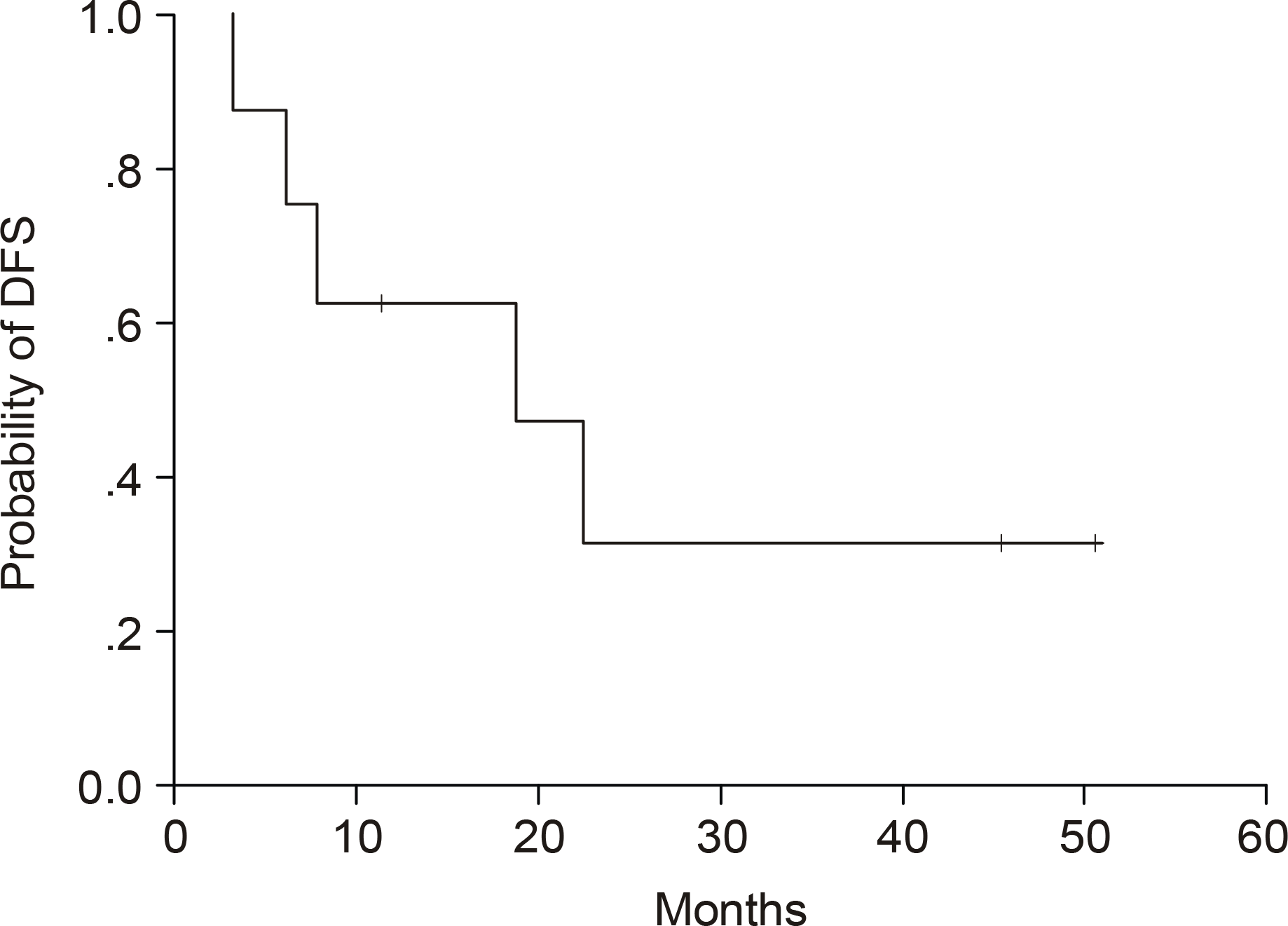
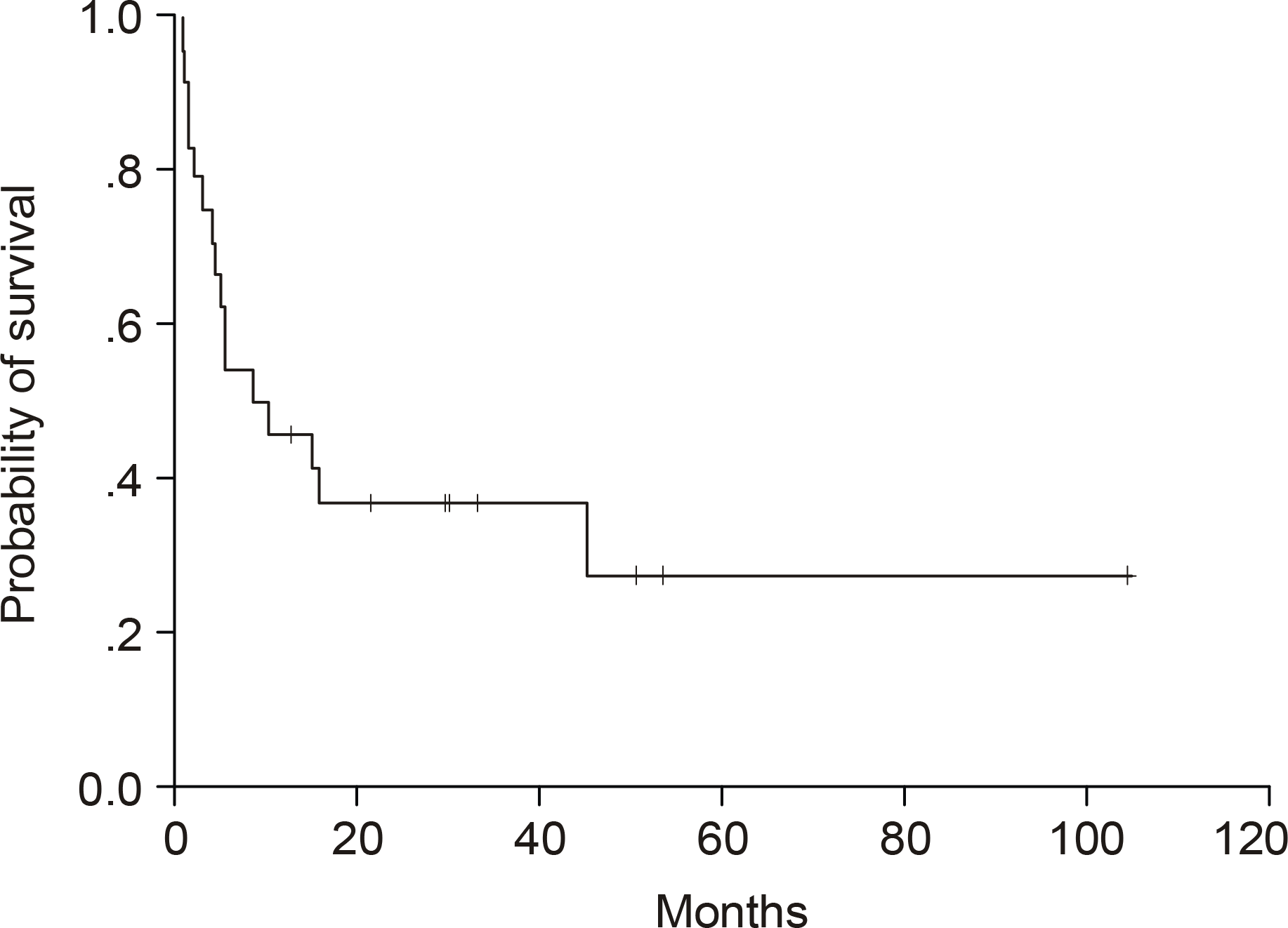
The predominant characteristics of the population were: median age 62 years (range, 32~81); M/F=18/6; nodal involvement 24/24 (100%); extranodal involvement, particularly bone marrow 16/20 (80%); skin involvement 6/24 (25%); B-symptoms 18/24 (75%) and advanced disease (stages III and IV) in 20/24 (83%). Twenty-three of the 24 patients received combination chemotherapy, with 8/23 (35%) of patients obtaining a CR. The median CR duration was 18.1 months. With a median follow-up of 40.9 months, the 5-year OS rate was 28%, with median survival of 8.7 months. According to a univariate analysis, an elevated LDH showed a tendency to negatively influence the survival.
Go to : 
REFERENCES
1). Ganesan TS, Dhaliwal HS, Dorreen MS, Stansfeld AG, Habeshaw JA, Lister TA. Angio-immunoblastic lymphadenopathy: a clinical, immunological and molecular study. Br J Cancer. 1987; 55:437–42.

2). Stansfeld AG, Diebold J, Noel H, et al. Updated Kiel classification for lymphomas. Lancet. 1988; 1:292–3.

3). Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994; 84:1361–92.
4). Harris NL, Jaffe ES, Diebold J, et al. The World Health Organization classification of neoplastic diseases of the haematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Histopathology. 2000; 36:69–86.

5). Siegert W, Agthe A, Griesser H, et al. Treatment of angioimmunoblastic lymphadenopathy (AILD)-type T-cell lymphoma using prednisone with or without the COPBLAM/IMVP-16 regimen. A multicenter study. Kiel Lymphoma Study Group. Ann Intern Med. 1992; 117:364–70.
6). Siegert W, Nerl C, Agthe A, et al. Angioimmunoblastic lymphadenopathy (AILD)-type T-cell lymphoma: prognostic impact of clinical observations and laboratory findings at presentation. The Kiel Lymphoma Study Group. Ann Oncol. 1995; 6:659–64.
7). Pautier P, Devidas A, Delmer A, et al. Angioimmunoblastic- like T-cell non Hodgkin's lymphoma: outcome after chemotherapy in 33 patients and review of the literature. Leuk Lymphoma. 1999; 32:545–52.
8). Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002; 99:627–33.

9). Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993; 328:1002–6.

10). Coleman M, Armitage JO, Gaynor M, et al. The COP- BLAM programs: evolving chemotherapy concepts in large cell lymphoma. Semin Hematol. 1988; 25(2 Suppl 2):23–33.
11). Kaneko Y, Maseki N, Sakurai M, et al. Characteristic karyotypic pattern in T-cell lymphoproliferative disorders with reactive “angioimmunoblastic lymphadenopathy with dysproteinemia-type” features. Blood. 1988; 72:413–21.

12). Schlegelberger B, Zhang Y, Weber-Matthiesen K, Grote W. Detection of aberrant clones in nearly all cases of angioimmunoblastic lymphadenopathy with dysproteinemia-type T-cell lymphoma by combined interphase and metaphase cytogenetics. Blood. 1994; 84:2640–8.

13). Schlegelberger B, Zwingers T, Hohenadel K, et al. Significance of cytogenetic findings for the clinical outcome in patients with T-cell lymphoma of angioimmunoblastic lymphadenopathy type. J Clin Oncol. 1996; 14:593–9.

14). Mahendran R, Grant JW, Hoggarth CE, Burrows NP. Angioimmunoblastic T-cell lymphoma with cutaneous involvement. J Eur Acad Dermatol Venereol. 2001; 15:589–90.

15). Martel P, Laroche L, Courville P, et al. Cutaneous involvement in patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Arch Dermatol. 2000; 136:881–6.
16). Pangalis GA, Moran EM, Nathwani BN, Zelman RJ, Kim H, Rappaport H. Angioimmunoblastic lymphadenopathy. longterm follow-up study. Cancer. 1983; 52:318–21.

17). Schetelig J, Fetscher S, Reichle A, et al. Longterm disease-free survival in patients with angioimmunoblastic T-cell lymphoma after high-dose chemotherapy and autologous stem cell transplantation. Haematologica. 2003; 88:1272–8.
18). Lindahl J, Kimby E, Bjorkstrand B, Christensson B, Hellstrom-Lindberg E. High-dose chemotherapy and APSCT as a potential cure for relapsing hemolysing AILD. Leuk Res. 2001; 25:267–70.

19). Jantunen E, D'Amore F. Stem cell transplantation for peripheral T-cell lymphoma. Leuk Lymphoma. 2004; 45:441–6.
20). Reimer P, Schertlin T, Rudiger T, et al. Myeloablative radiochemotherapy followed by autologous peripheral blood stem cell transplantation as first-line therapy in peripheral T-cell lymphoma: first results of a prospective multicenter study. Hematology J. 2004; 5:304–11.
21). Kang YK, Kim BS, Kim TW, et al. Clinicopathologic characteristics of Korean Non-Hodgkin's Lymphomas based on REAL classitication. J Korean Cancer Assoc. 1999; 31:641–52.
22). Ko YH, Kim CW, Park CS, et al. REAL classification of malignant lymphomas in the Republic of Korea: incidence of recently recognized entities and changes in clinicopathologic features. Hematolymphoreticular Study Group of the Korean Society of Pathologists. Revised European-American lymphoma. Cancer. 1998; 83:806–12.
23). The Non-Hodgkin's Lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. Blood. 1997; 89:3909–18.
Go to : 
Table 1.
Relative frequency of histologic subtypes of NHLs according to REAL classification∗
Table 2.
Clinical characteristics of the AITL patients (N=24)
| N | % | |
|---|---|---|
| Age median: 62yr (32~81) | ||
| ≤60yr | 10 | 42 |
| >60yr | 14 | 58 |
| Sex | ||
| Males | 18 | 75 |
| Females | 6 | 25 |
| Stage | ||
| I~II | 4 | 17 |
| III~IV | 20 | 83 |
| Performance status | ||
| 1~2 | 18 | 75 |
| 3~4 | 6 | 25 |
| B-symptoms | ||
| Present | 18 | 75 |
| Absent | 6 | 25 |
| LN enlargement | 24 | 100 |
| Hepatosplenomegaly | 16 | 66 |
| Skin | ||
| Rash | 5 | 20 |
| Nodules | 1 | 4 |
| Extranodal involvement | ||
| Bone marrow | 16/20∗ | 80 |
| Pleural effusion | 9 | 38 |
| Ascites | 3 | 13 |
| Cerebrospinal fluid | 0 | 0 |
| IPI | ||
| Low risk | 1 | 4 |
| Low-intermediate | 3 | 13 |
| High-intermediate | 7 | 29 |
| High | 13 | 54 |
Table 3.
Laboratory findings of the AITL patients (N=24)
| N | % | |
|---|---|---|
| Hemoglobin (g/dL) | ||
| <10 | 10 | 42 |
| ≥10 | 14 | 58 |
| Hypereosinophilia (>500/μL) | 8 | 33 |
| Polyclonal hypergammoglobulinemia (>13g/dL) | 8/12 | 67∗ |
| LDH (IU/L) | ||
| Normal (<225) | 3 | 12 |
| ~300 | 7 | 29 |
| 301~400 | 7 | 29 |
| 401~500 | 3 | 13 |
| >500 | 4 | 17 |
Table 4.
Response according to initial therapy
| First-line therapy | N | CR N (%) | PR N (%) | Median CR duration | Relapse |
|---|---|---|---|---|---|
| Month | N (%) | ||||
| Chemotherapy | 23 | 8 (35) | 4 (17%) | ||
| Anthracycline-based∗ | 16 | 8 (50) | 2 (13%) | 18.1 | 5/8 (62) |
| Others† | 7 | 0 (0) | 2 (29%) |
Table 5.
Prognostic factor rs influencing overa all survival
Table 6.
Comparision of patient characteristics and treatment outcomes




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download