Abstract
Background
Hereditary dysfibrinogenemia is a rare cause of venous thromboembolism. Hereditary thrombophilia is diagnosed in about 10% of patients with thromboembolism, with the prevalence diagnosed increasing with the development of molecular biological method.
Methods
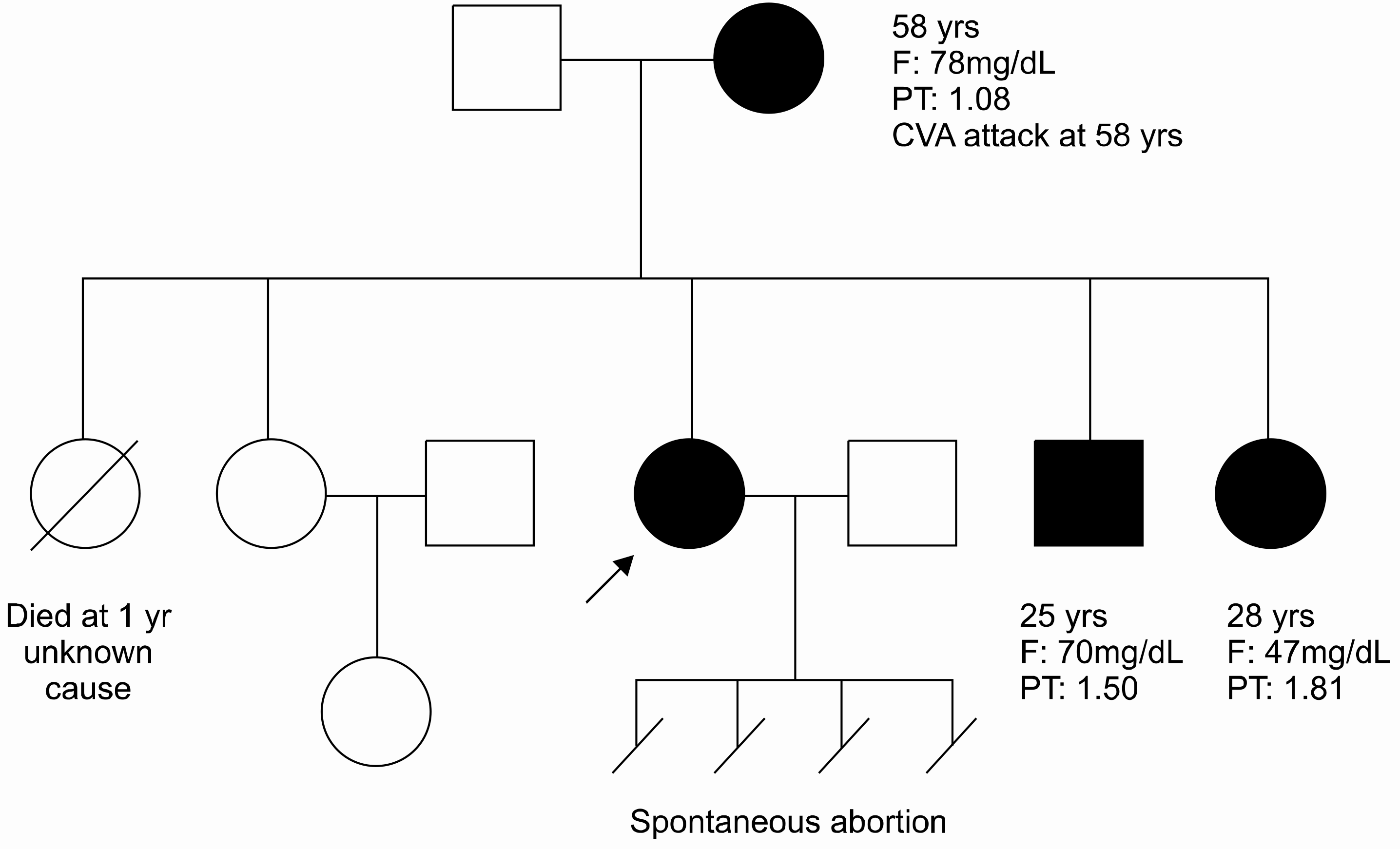
A 27-year-old woman was strongly suspected to have hereditary dysfibrinogenemia; therfore, an analysis of the molecular structure of the purified fibrinogen was performed.
Results
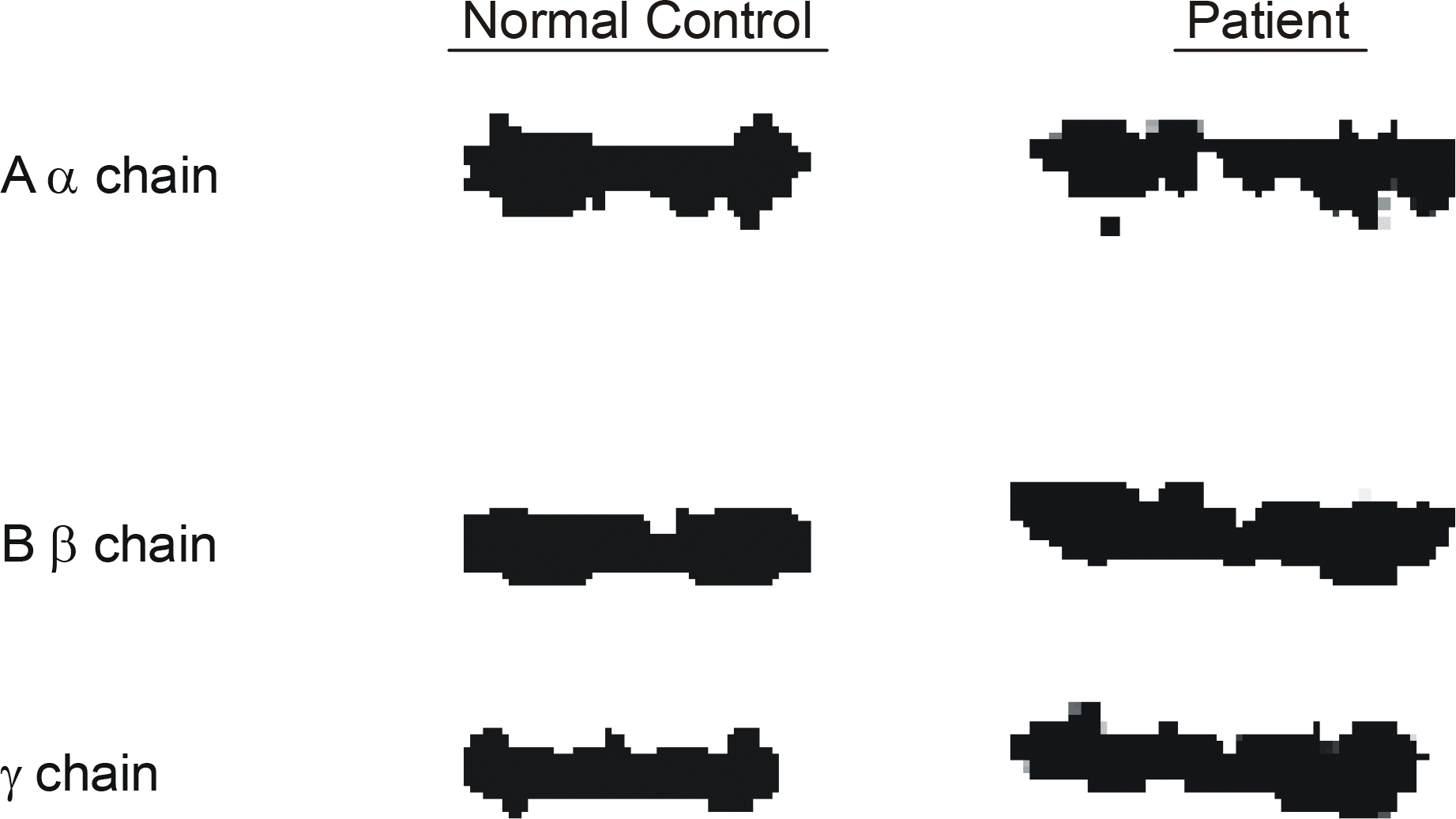
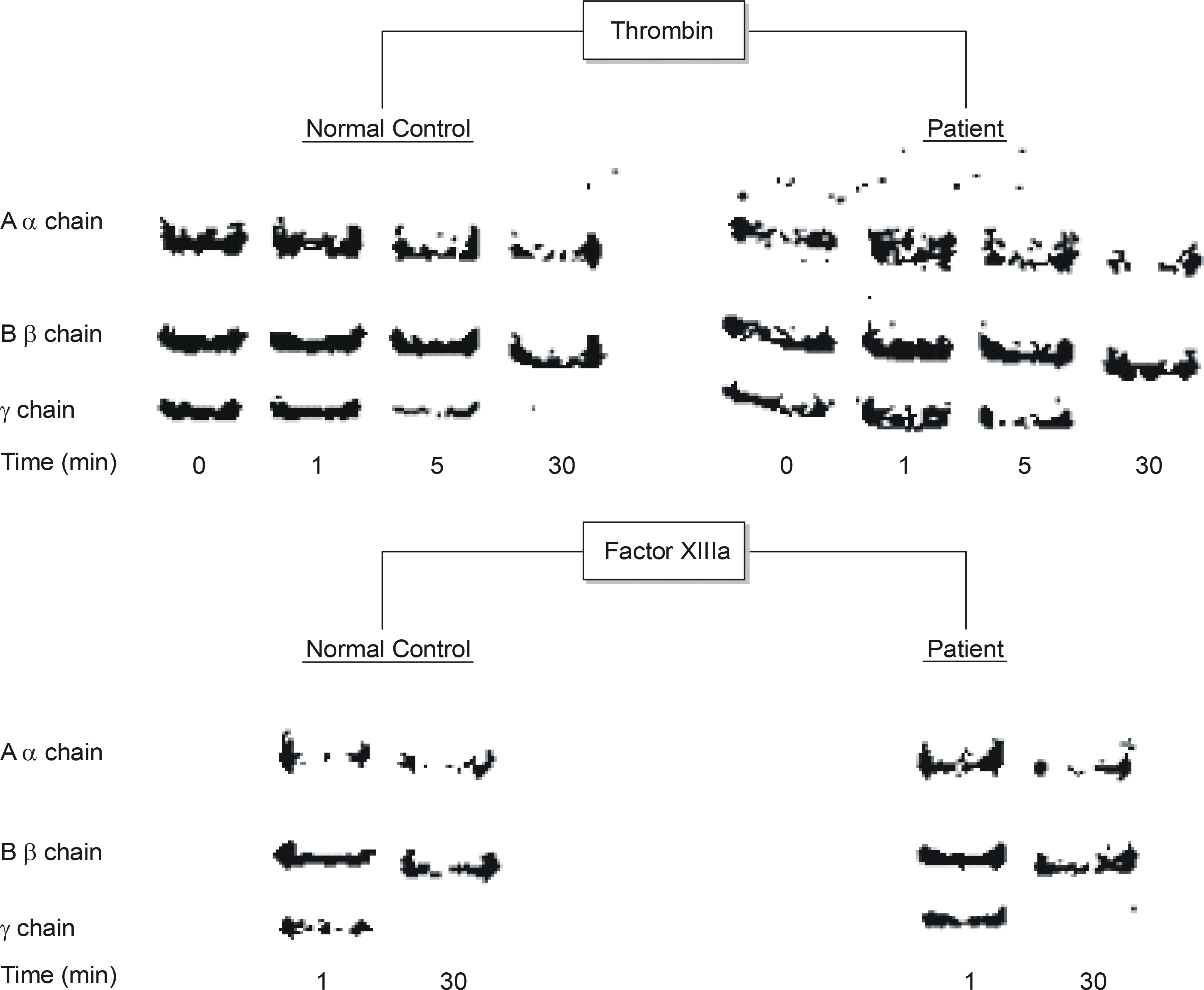
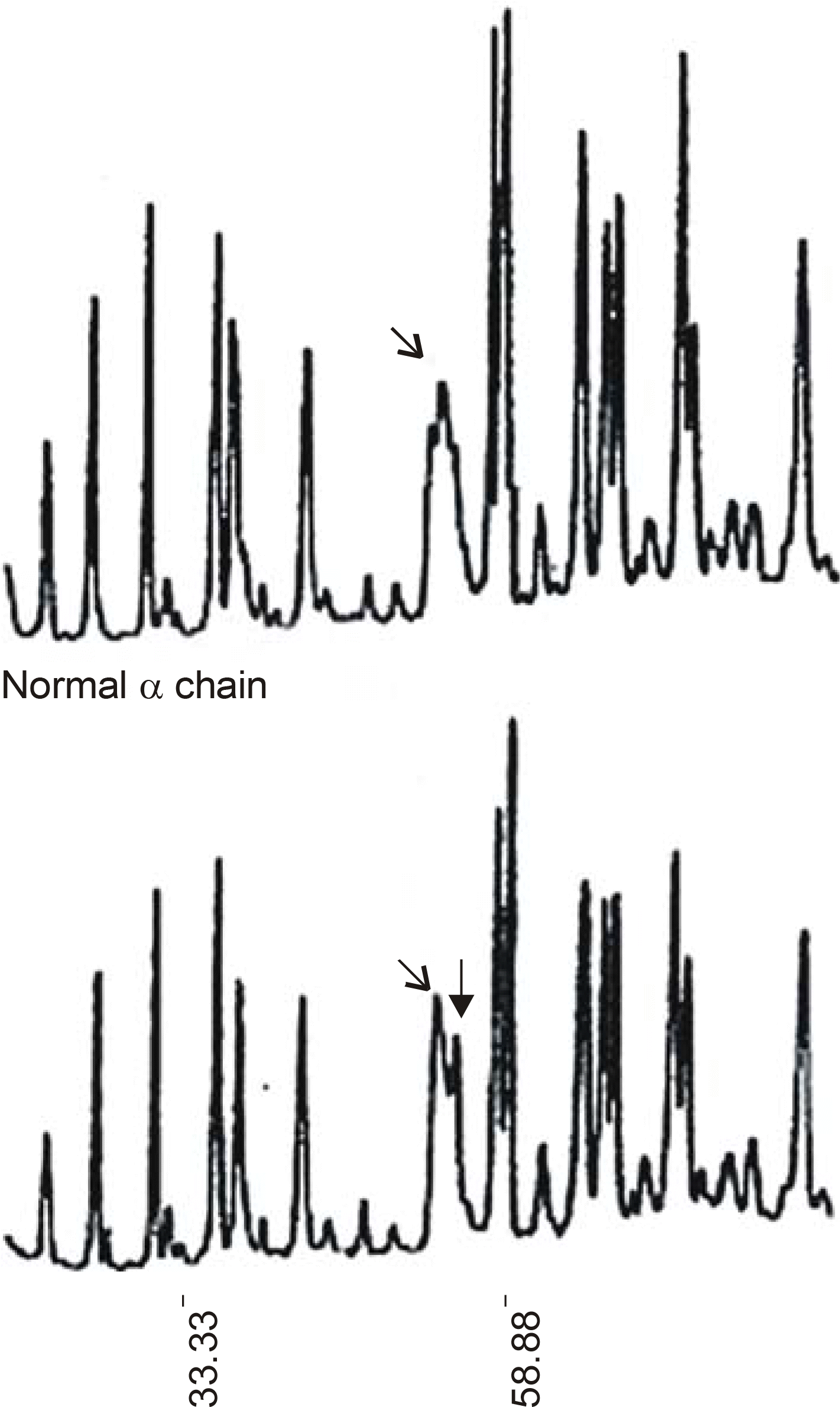
An SDS-PAGE analysis of the purified fibrinogen revealed no abnormal finding. The purified fibrinogen was treated with thrombin or coagulation factor XIII, and the products show no difference between the normal and patient's specimen on SDS-PAGE analysis. However, an HPLC analysis showed an additional abnormal peak prior to the normal fibrinopeptid A peak.
Go to : 
REFERENCES
1). Malm J, Laurell M, Nilsson IM, Dahlback B. Thromboembolic disease-critical evaluation of laboratory investigation. Thromb Haemost. 1992; 68:7–13.
3). Mosesson MW. Hereditary abnormalities of fibrinogen. Beutler E, Lichtman MA, Coller BS, Kipps TJ, Seligsohn U, editors. Williams hematology. 6th ed.New York: McGraw-Hill Co;2006. p. 1659–71.
4). Terukina S, Yamazumi K, Okamoto K, Yamashita H, Ito Y, Matsuda M. Fibrinogen Kyoto III: a congenital dysfibrinogen with a gamma aspartic acid-330 to tyrosine substitution manifesting impaired fibrin monomer polymerization. Blood. 1989; 74:2681–7.

5). Miyata T, Furukawa K, Iwanaga S, Takamatsu J, Saito H. Fibrinogen Nagoya, a replacement of glutamine-329 by arginine in the gamma-chain that impairs the polymerization of fibrin monomer. J Biochem (Tokyo). 1989; 105:10–4.
6). Yoshida N, Hirata H, Morigami Y, et al. Characterization of an abnormal fibrinogen Osaka V with the replacement of gamma-arginine 375 by glycine. The lack of high affinity calcium binding to D-domains and the lack of protective effect of calcium on fibrinolysis. J Biol Chem. 1992; 267:2753–9.

7). Matsuda M, Baba M, Morimoto K, Nakamikawa C. “Fibrinogen Tokyo II”. An abnormal fibrinogen with an impaired polymerization site on the aligned DD domain of fibrin molecules. J Clin Invest. 1983; 72:1034–41.

8). Matsuda M, Saeki E, Kasamatsu A, Nakamikawa C, Manabe S, Samejima Y. Fibrinogen Kawaguchi: an abnormal fibrinogen characterized by defective release of fibrinopeptide A. Thromb Res. 1985; 37:379–90.
9). Cunningham MT, Brandt JT, Laposata M, Olson JD. Laboratory diagnosis of dysfibrinogenemia. Arch Pathol Lab Med. 2002; 126:499–505.

10). Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970; 227:680–5.
11). Francis CW, Marder VJ, Martin SE. Detection of circulating crosslinked fibrin derivatives by a heat extraction-SDS gradient gel electrophoretic technique. Blood. 1979; 54:1282–95.

12). Vanfleteren JR, Raymackers JG, Van Bun SM, Me-heus LA. Peptide mapping and microsequencing of proteins separated by SDS-PAGE after limited in situ acid hydrolysis. Biotechniques. 1992; 12:550–2. 554, 556-7.
13). Haverkate F, Samama M. Familial dysfibrinogenemia and thrombophilia. Report on a study of the SSC Subcommittee on fibrinogen. Thromb Haemost. 1995; 73:151–61.
15). Matsuda M. Structure and function of fibrinogen inferred from hereditary dysfibrinogens. Int J Hematol. 2000; 72:436–47.

16). Koopman J, Haverkate F, Grimbergen J, Egbring R, Lord ST. Fibrinogen Marburg: a homozygous case of dysfibrinogenemia, lacking amino acid Aα461-610. Blood. 1992; 80:1972–9.
17). Soria J, Soria C, Hedner U, Nilsson IM, Bergqvist D, Samama M. Episodes of increased fibronectin level observed in a patient suffering from recurrent thrombosis related to congenital hypodysfibrino genemia (fibrinogen Malmoe). Br J Haematol. 1985; 61:727–38.
18). Nilsson IM, Nilehn JE, Cronberg S. Hypofibrinogenemia and massive thrombosis. Acta Med Scand. 1966; 180:65–76.
19). Ness PM, Budzynski AZ, Olexa SA, Rodvien R. Congenital hypofibrinogenemia and recurrent placental abruption. Obstet Gynecol. 1983; 61:519–23.
20). Loreth RM, Meyer M, Albert FW. Fibrinogen Kai-serslauten III: a new case of congenital dysfibrinogenemia with A alpha 16 arg→cys substitution. Haemostasis. 2001; 31:12–7.
21). Mathonnet F, Peltier JY, Roda L, et al. Three new cases of dysfibrinogenemia: Poissy III, Saint-Germain I and Tahiti. Thromb Res. 2001; 103:201–7.
22). Lounes KC, Soria C, Mirshahi SS, et al. Fibrinogen Ales: a homozygous case of dysfibrinogenemia (gamma-Asp (330)→Val) characterized by a defective fibrin polymerization site a. Blood. 2000; 96:3473–9.
23). Tarumi T, Martincic D, Thomas A, Janco R, Hudson M, Baxter P, Gailani D. Familial thrombophilia associated with fibrinogen Paris V: Dusart syndrome. Blood. 2000; 96:1191–3.

24). Kim I, Park S, Lee J, et al. Hereditary thrombophilia in Korea. Korean J Med. 1996; 51:732–42.
25). Siebenlist KR, Mosesson MW, Meh DA, DiOrio JP, Albrecht RM, Olson JD. Coexisting dysfibrinogenemia (gamma R275C) and factor V Leiden deficiency associated with thromboembolic disease (fibrinogen Cedar Rapids). Blood Coagul Fibrinolysis. 2000; 11:293–304.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download