Abstract
Background
Ever since medical professionals have recognized the important role of ADAMTS-13 in the pathogenesis of TTP, several methods to diagnosis the activity of ADAMTS-13 in the plasma of TTP patients haves been developed. However these assays have not been widely used in practice because they are cumbersome and they require several days to complete. In this study we examine the new, rapid ADAMTS-13 activity assay that uses fluorescence resonance energy transfer and we compared it with the conventional assay to determine its diagnostic advantage.
Methods
Seven TTP patients were compared with 60 healthy controls. The plasma ADAMTS-13 activity was measured using the fluorescence-quenching substrate assay method. The results were compared with the results of performing multimer analysis of SDS-agarose gel electrophoresis.
Results
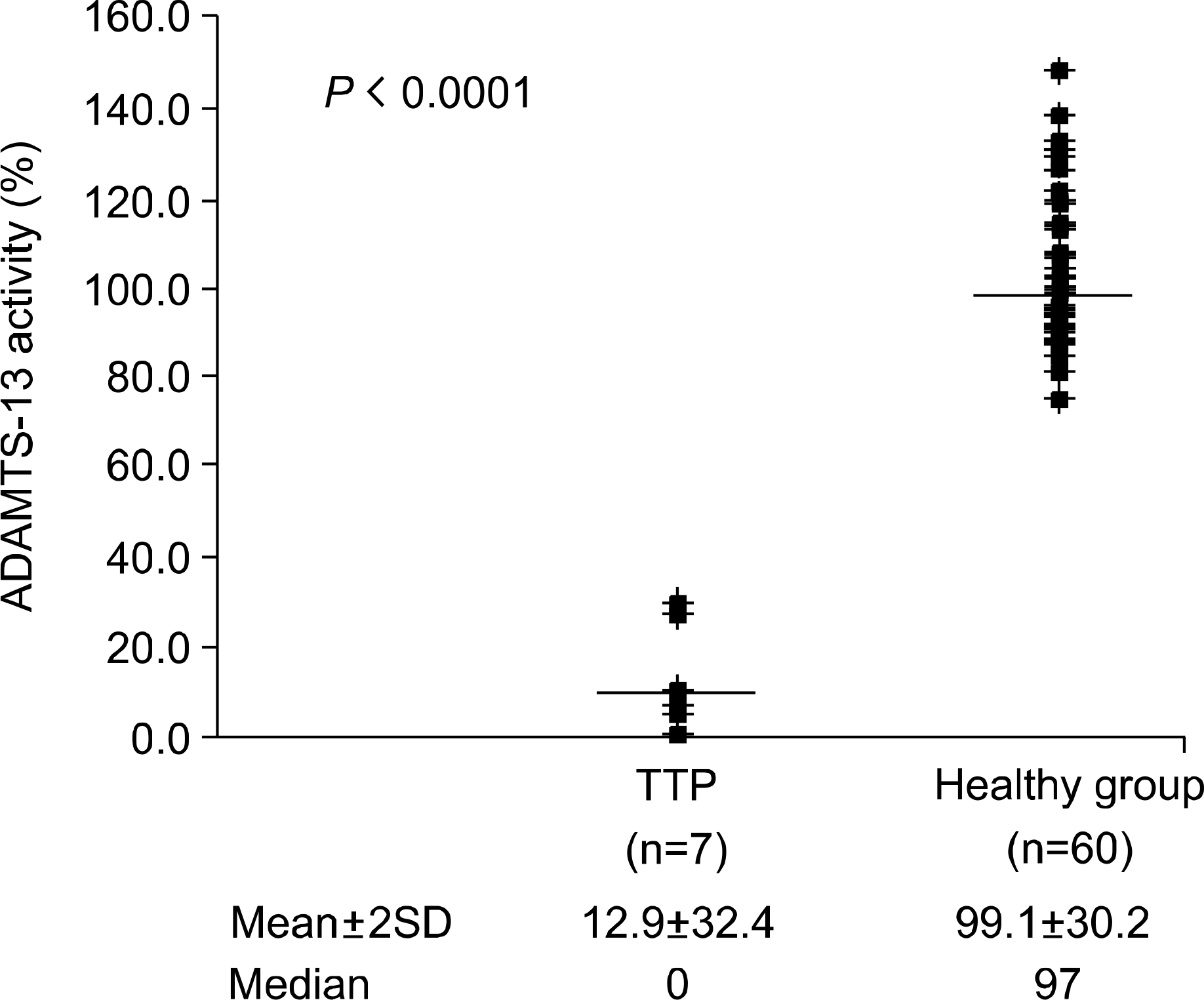
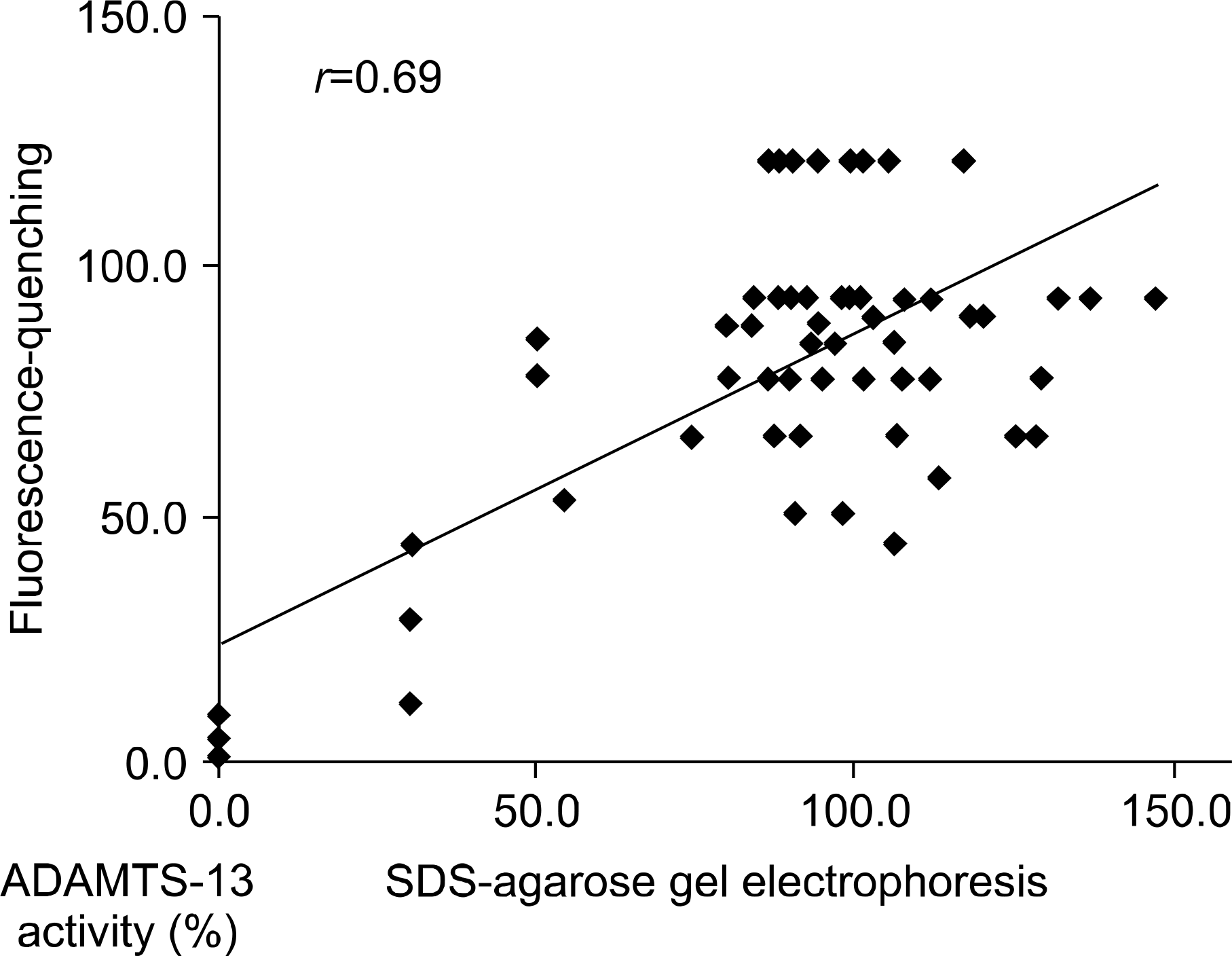
It took only 2 hour to complete the fluorescence-quenching substrate assay. The median ADAMTS-13 activity using the fluorescence-quenching substrate was 5.9% (range: 0~29.9%) for the patient group and 99.1% (range: 74.4~143.3%) for the healthy group, respectively. The median ADAMTS-13 activity using multimer analysis of SDS-agarose gel electrophoresis was 5.6% (range: 1.6~ 28.8%) for the patients group and 87.7% (range: 44.1~120.9%) for the healthy group, respectively. The ADAMTS-13 activities of the two assays were well correlated (correlation coefficient: 0.69).
Go to : 
REFERENCES
1). Moschcowitz E. Hyaline thrombosis of the terminal arterioles and capillaries: a hitherto undescribed disease. Proc NY Pathol Soc. 1924; 24:21–4.
2). Moake JL, Rudy CK, Troll JH. Unusually large plasma factor VIII: von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med. 1982; 307:1432–5.

3). George JN. How I treat patients with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Blood. 2000; 96:1223–9.

4). Zheng XL, Kaufman RM, Goodnough LT, Sadler JE. Effect of plasma exchange on plasma ADAMTS 13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidio-pathic thrombotic thrombocytopenic purpura. Blood. 2004; 103:4043–9.
5). Furlan M, Robles R, Lamie B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996; 87:4223–34.

6). Tsai HM. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 1996; 87:4235–44.

7). Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005; 129:93–100.

8). Gerritsen HE, Turecek PL, Schwarz HP, Lammle B, Furlan M. Assay of von Willebrand factor (vWF)-cleaving protease based on decreased collagen binding affinity of degraded vWF: a tool for the diagnosis of thrombotic thrombocytopenic purpura (TTP). Thromb Haemost. 1999; 82:1386–9.
9). Obert B, Tout H, Veyradier A, Fressinaud E, Meyer D, Girma JP. Estimation of the von Willebrand factor-cleaving protease in plasma using monoclonal antibodies to vWF. Thromb Haemost. 1999; 82:1382–5.

10). Bohm M, Vigh T, Scharrer I. Evaluation and clinical application of a new method for measuring activity of von Willebrand factor-cleaving metalloprotease (ADAMTS-13). Ann Hematol. 2002; 81:430–5.

11). Studt JD, Bohm M, Budde U, Girma JP, Varadi K, Lammle B. Measurement of von Willebrand factor-cleaving protease (ADAMTS-13) activity in plasma: a multicenter comparison of different assay methods. J Thromb Haemost. 2003; 1:1882–7.

12). Mannucci PM, Canciani MT, Forza I, Lussana F, Lattuada A, Rossi E. Changes in health and disease of the metalloprotease that cleaves von Willebrand factor. Blood. 2001; 98:2730–5.

13). Jang MJ, Chong SY, Lee SJ, Kim NK, Kang MS, Oh D. Changes of the activity of ADAMTS-13 in aging, pregnancy and disease. Korean J Hematol. 2004; 39:71–7.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download