Abstract
Background
Pernicious anemia is the most common cause of vitamin B12 deficiency in western populations, but to date, only case reports or small series dealing with this malady have been reported in Korea. This study describes the clinical characteristics of pernicious anemia in Koreans.
Methods
We retrospectively analyzed the clinical data for twenty-two Korean patients with pernicious anemia who were diagnosed during the period from 1995 to 2004 at Chungnam National University Hospital.
Results
Only two patients were diagnosed before 2000. The median age of the patients was 66 years and the male/female ratio was 1.8. Anemia-associated discomfort was the most common symptom (95.5%); this was followed by gastrointestinal and neurological symptoms (77.2% and 50.0%, respectively). Autoimmune disorders were found in five patients (22.7%). The median hemoglobin level was 7.0g/dL (range: 3.1~11.8g/dL) and pancytopenia was found in 12 patients (54.5%). The median serum vitamin B12 Level was 26pg/mL (range: 12~189pg/mL). Fifteen (78.9%) and eight (42.1%) of the 19 patients who underwent tests for antibodies were positive for anti-intrinsic factor and anti-parietal cell antibody, respectively. Nineteen of 21 patients who were treated with intramuscular cobalamin recovered from their cytopenia within 3 months. The gastrointestinal symptoms resolved completely for all the patients, while neurological symptoms remained for some of the patients.
Go to : 
REFERENCES
1). Babior BM, Bunn HF. Megaloblastic anemia. Kasper DL, Braunwald E, Fauci AS, editors. Harrison's principles of internal medicine. 16th ed.New York: McGrqw Hill Co;2006. p. 601–7.
3). Borch K, Liedberg G. Prevalence and incidence of pernicious anemia. An evaluation for gastric screening. Scand J Gastroenterol. 1984; 19:154–60.
4). Park AJ, Kim HT, Kim SS, Kim SG, Son YA. Clinical and laboratory diagnosis of pernicious anemia (one case report). Korean J Hematol. 1983; 18:217–23.
5). Lee DG, Do IH, Kim DW, Park JI, Chung SY, Moon SK. A case of pernicious anemia associated with chronic atropic gastritis. Korean J Hematol. 1987; 22:115–20.
6). Hahn JS, Park JO, Lee S, et al. A case of pernicious anemia with autoimmune hemolytic anemia. Korean J Hematol. 1995; 30:315–20.
7). Lee JS, Jeon JJ, Suh JI, et al. Clinical consideration of vitamin B12 deficiency megaloblastic anemia. Korean J Hematol. 1994; 29:259–67.
8). Song HH, Kwon JH, Kim JH, et al. Causes and clinical features of vitamin B12 deficiency megaloblastic anemia. Korean J Hematol. 2004; 39:243–8.
9). Castle WB. Current concept of pernicious anemia. Am J Med. 1970; 48:541–8.
10). Carmel R, Green R, Rosenblatt DS, Watkins D. Update on cobalamin, folate, and homocysteine. Hematology. 2003. 62–81.

11). Carmel R. Pepsinogens and other serum markers in pernicious anemia. Am J Clin Pathol. 1988; 90:442–5.

12). Andres E, Loukili NH, Noel E, et al. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ. 2004; 171:251–9.

13). Chanarin I. Historical review: a history of pernicious anaemia. Br J Haematol. 2000; 111:407–15.
14). Toh BH, Alderuccio F. Pernicious anemia. Autoimmunity. 2004; 37:357–61.
15). Andres E, Perrin AE, Demangeat C, et al. The syndrome of food-cobalamin malabsorption revisited in a department of internal medicine. A monocentric cohort study of 80 patients. Eur J Intern Med. 2003; 14:221–6.
16). Rice L. Food-cobalamin malabsorption: a usual cause of vitamin B12 deficiency. Arch Intern Med. 2000; 160:2061–2.
17). Carmel R. Reassessment of the relative prevalence of antibodies to gastric parietal cell and to intrinsic factor in patients with pernicious anaemia: influence of patients age and race. Clin Exp Immunol. 1992; 89:74–7.
18). Carmel R. Malabsorption of food cobalamin. Bail-lieres Clin Haematol. 1995; 8:639–55.
19). Antony AC. Megaloblastic anemias. Hoffman R, Benz EJ, Shattil SJ, editors. Hematology: basic principles and practice. 4th ed.New York: Churchill Livingstone;2006. p. 533–4.

20). Perez-Perez GI. Role of Helicobacter pylori infection in the development of pernicious anemia. Clin Infect Dis. 1997; 25:1020–2.
Go to : 
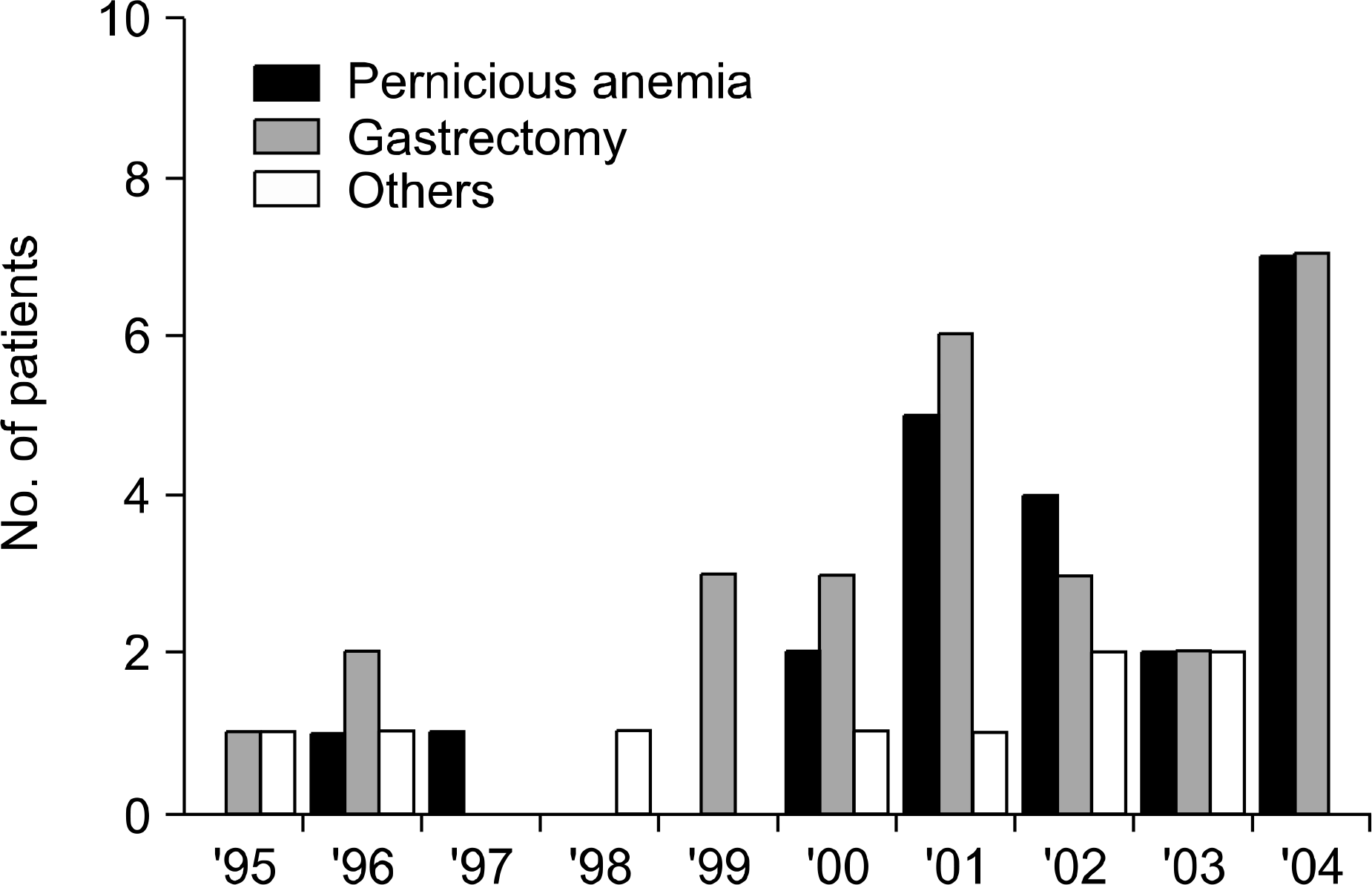 | Fig. 1.Annual distribution of the numbers of newly diagnosed patients with megaloblastic anemia associated with cobalamin deficiency. |
Table 1.
Causes of vitamin B12 deficiency in megaloblastic anemia (n=58)
| Causes | No. of patients (%) |
|---|---|
| Pernicious anemia | 22 (37.9) |
| Gastrectomy | 27 (46.6) |
| Others | 9 (15.5) |
Table 2.
Patient characteristics (n=22)
Table 3.
Symptoms and signs (n=22)
Table 4.
Laboratory findings at the time of diagnosis
Table 5.
Gastroscopic and histologic findings
Table 6.
Responses to cobalamin therapy (n=21)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download