Abstract
Background
Acute promyelocytic leukemia (APL) is distinguished from other forms of leukemia by its association with bleeding diatheses. All-trans retinoic acid (ATRA) and arsenic trioxide (As2O3), which have been effectively used in the treatment of the APL, promptly improve coagulation/bleeding syndromes by regulating the expressions of tissue factor (TF) and thrombomodulin (TM). We have previously shown a novel activity of auranofin (AF), in that it induced apoptosis and differentiation of NB4 cells. To study whether AF also possesses similar anticoagulant effects to those of ATRA and As2O3, its effects on the expressions of TM and TF were investigated.
Methods
NB4 cells derived from APL were incubated with 1μM of AF. After incubation for 12, 24 and 48 hours, the AF-regulated expressions of TM and TF were analyzed by RT-PCR, Northern blot and Western blot. The assay for the TM antigen on the cell surface was performed using a flow cytometry.
Results
The expression of the TM gene was increased for upto 12 hours after the AF treatment, but no change was observed in the expression of the TF gene. Western blot analysis also demonstrated that AF increased the level of TM proteinin a time-dependent manner. FACS data showed the TM antigen on the cell surface to gradually increase for upto 48 hours in AF-treated cells.
Go to : 
REFERENCES
1). Mistry AR, Pedersen EW, Solomon E, Grimwade D. The molecular pathogenesis of acute promyelocytic leukaemia: implications for the clinical management of the disease. Blood Rev. 2003; 17:71–97.

2). Dombret H, Scrobohaci ML, Ghorra P, et al. Coagulation disorders associated with acute promyelocytic leukemia: corrective effect of all-trans retinoic acid treatment. Leukemia. 1993; 7:2–9.
3). Barbui T, Finazzi G, Falanga A. The impact of all-trans-retinoic acid on the coagulopathy of acute promyelocytic leukemia. Blood. 1998; 91:3093–102.

4). Chen GQ, Shi XG, Tang W, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997; 89:3345–53.
5). Agis H, Weltermann A, Mitterbauer G, et al. Successful treatment with arsenic trioxide of a patient with ATRA-resistant relapse of acute promyelocytic leukemia. Ann Hematol. 1999; 78:329–32.

6). Koyama T, Hirosawa S, Kawamata N, Tohda S, Aoki N. All-trans retinoic acid upregulates thrombomodulin and downregulates tissue-factor expression in acute promyelocytic leukemia cells: distinct expression of thrombomodulin and tissue factor in human leukemic cells. Blood. 1994; 84:3001–9.

7). Zhao W, Wang H, Wang X, et al. Effects of all-trans-retinoic acid and arsenic trioxide on the hemostatic disturbance associated with acute promyelocytic leukemia. Thromb Res. 2001; 102:197–204.

8). Snyder RM, Mirabelli CK, Crooke SJ. The cellular pharmacology of auranofin. Semin Arthritis Rheum. 1987; 17:71–80.

9). Jeon KI, Jeong JY, Jue DM. Thiol-reactive metal compounds inhibit NF-kB activation by blocking IkB kinase. J Immunol. 2000; 164:5981–9.
10). Kim IS, Jin JY, Lee IH, Park SJ. Auranofin induces apoptosis and when combined with retinoid acid enhances differentiation of acute promyelocytic leukaemia cells in vitro. Br J Pharmacol. 2004; 142:749–55.
11). Park SJ, Kim IS. The role of p38 MAPK activation for auranofin-induced apoptosis of human promyelocytic leukaemia HL-60 cells. Br J Pharmacol 2005, in press.
12). Lee IH, Lee JH, Lee MJ, Lee SY, Kim IS. Involvement of CCAAT/enhancer-binding protein alpha in haptoglobin gene expression by all-trans-retinoic acid. Biochem Biophys Res Commun. 2002; 294:956–61.
13). Shibakura M, Koyama T, Saito T, et al. Anticoagulant effects of synthetic retinoids mediated via different receptors on human leukemia and umbilical vein endothelial cells. Blood. 1997; 90:1545–51.

14). Hashimoto K, Whitehurst CE, Matsubara T, Hirohata K, Lipsky PE. Immunomodulatory effects of therapeutic gold compounds. J Clin Invest. 1992; 89:839–48.
15). Gromer S, Arscott LD, Williams CH Jr, Schirmer RH, Becker K. Human placenta thioredoxin reductase. Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J Biol Chem. 1998; 273:20096–101.
16). Rigobello MP, Scutari G, Boscolo R, Bindoli A. Induction of mitochondrial permeability transition by auranofin, a gold (I)-phosphine derivative. Br J Pharmacol. 2002; 136:1162–8.
17). Rigobello MP, Scutari G, Folda A, Bindoli A. Mitochondrial thioredoxin reductase inhibition by gold (I) compounds and concurrent stimulation of permeability transition and release of cytochrome c. Biochem Pharmacol. 2004; 67:689–96.
Go to : 
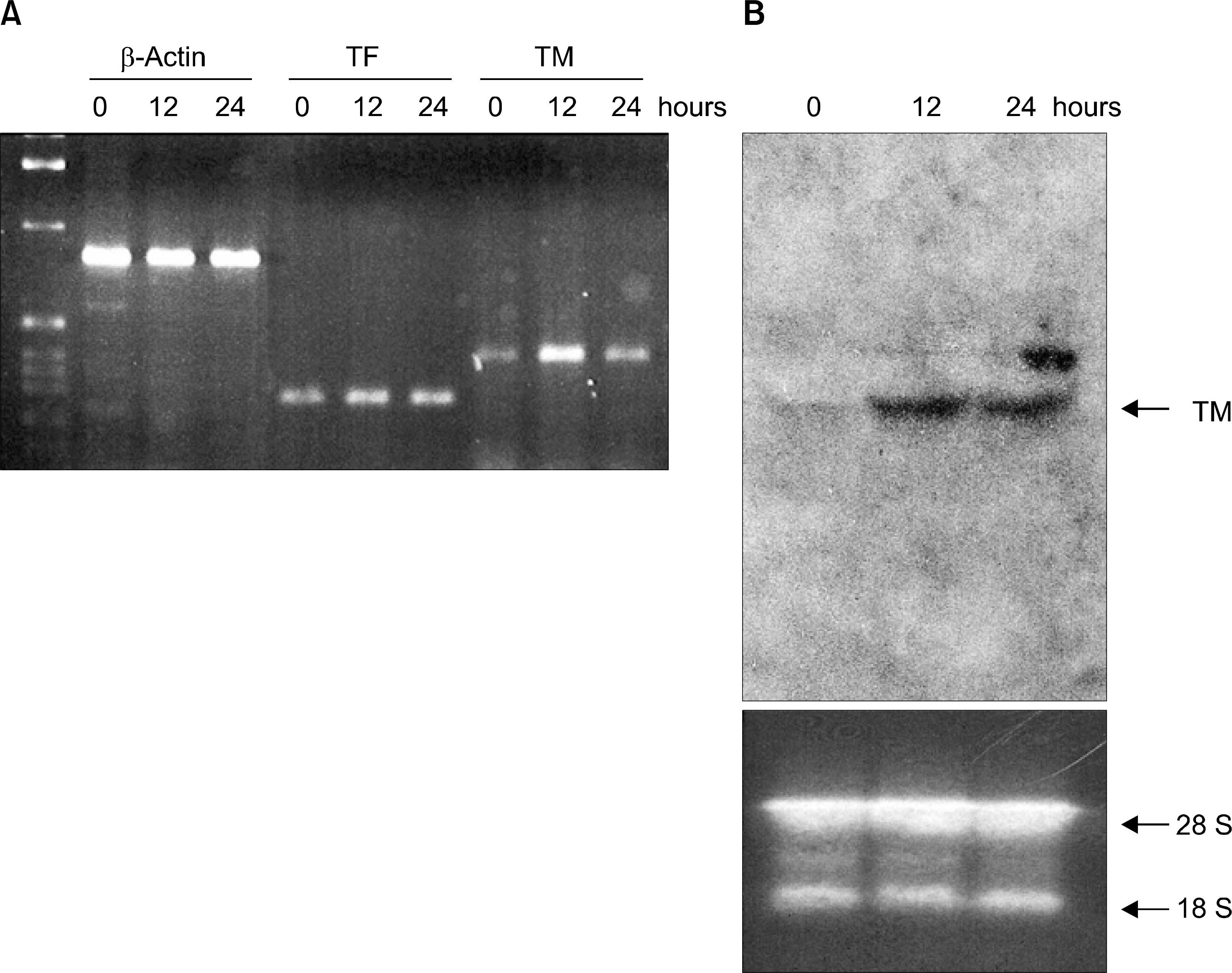 | Fig. 2.The effect of AF on TM and TF gene expressions. NB4 cells were treated with 1μM of AF for 12 and 24 hours, and then total cellular RNA was isolated using RNAzol B solution. (A) To analyze TM and TF gene expressions, RT-PCR was performed as described in materials and methods. Equal amounts of RNA used in each sample were evaluated by detecting β-actin expression. (B) Northern blot analysis. 20μg of total RNA per lane was loaded and electrophoresed on a 1.2% agarose/formaldehyde gel. DIG-labeled PCR product of human TM gene was used as a probe. 28 S and 18 S ribosomal RNA shown in the EtBr-stained gel indicate the equal amount of loaded RNA samples. The results shown (A and B) are representative of at least two independent experiments. |
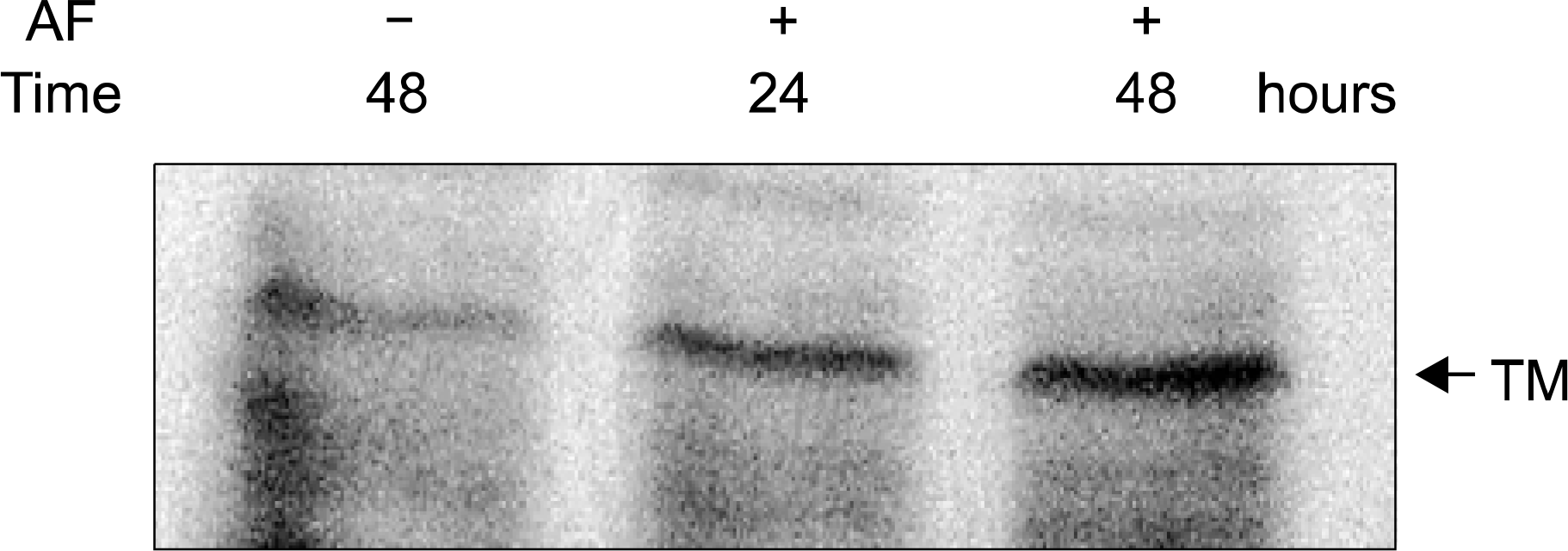 | Fig. 3.The enhancement of TM antigen level by AF. After treatment with 1μM of AF for the indicated time, 50μg of cellular proteins were subjected to SDS-polyacrylamide gel electrophoresis and the TM protein was analyzed by Western blot by using anti-TM antibody. The blot shown is representative of three independent experiments. |
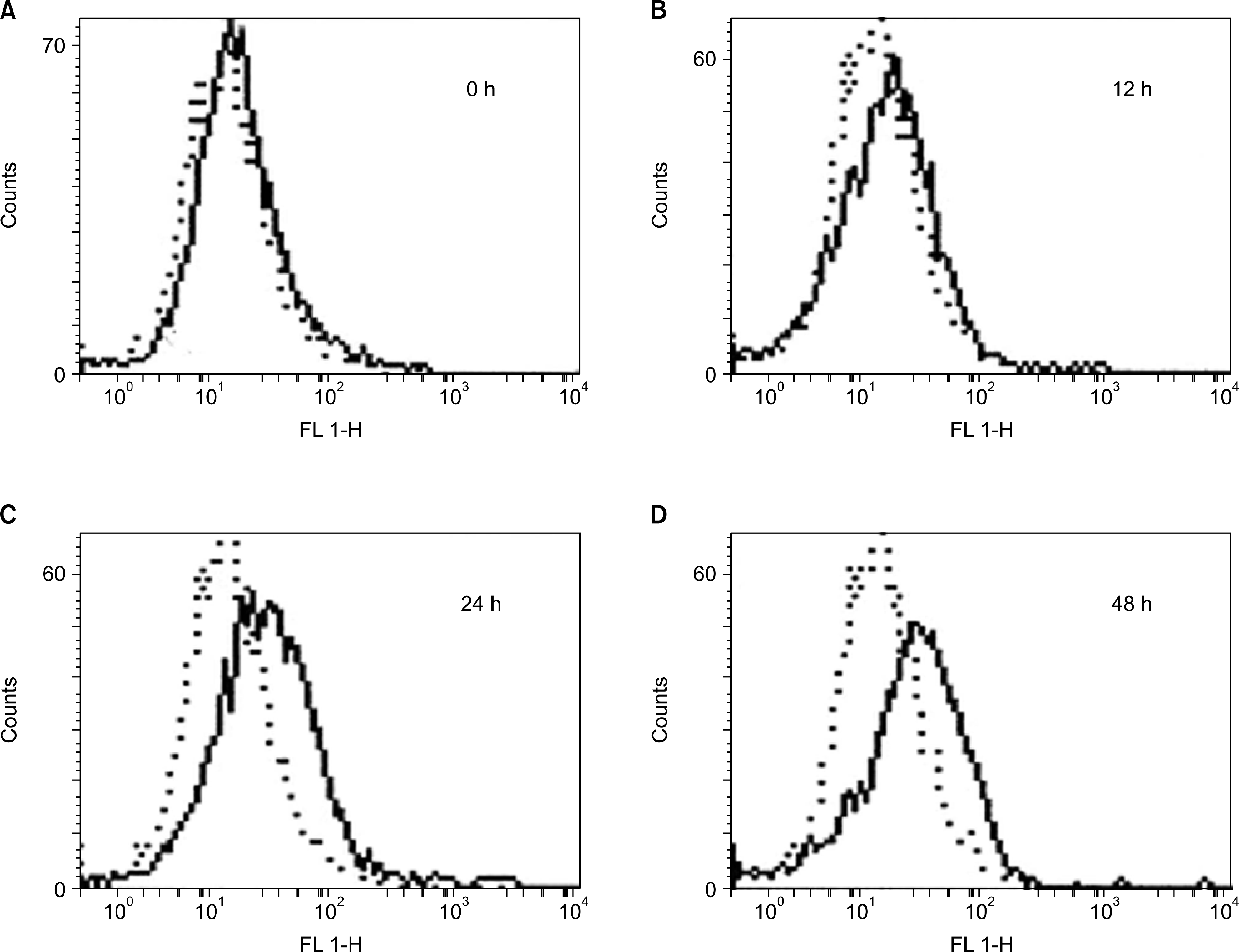 | Fig. 4.The flow cytometric analysis of surface TM antigen. The NB4 cells were treated with 1μM of AF for 0, 12, 24 and 48 hours. After that, the cells were sequentially incubated with anti-TM antibody and FITC -conjugated anti-rabbit IgG, and analyzed by a flow cytometer. Note that the population of cells with FITC-fluorescence was elevated in a time-dependent manner. Dashed line is for isotype control by using rabbit IgG instead of anti-TM antibody. The data shown represent a typical result obtained from two independent experiments. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download