Abstract
Spindle cell carcinoma (SpCC) is referred to as a variant of oral squamous cell carcinoma. It is also known as "sarcomatoid squamous cell carcinoma" because it consists of normal squamous carcinoma cells with spindle-shaped cells that appear similar to a sarcoma. The term, "second primary tumor" (SPT) or "double primary tumor", is proposed for a second tumor that develops independently from the first. SPTs can present as either synchronous or metachronous lesions. Synchronous SPTs are defined as tumors occurring simultaneously or within 6 months after the first tumor. The patient in this case, whose primary tumor was in the tongue, was diagnosed with SpCC with metastases to both neck lymph nodes. This case also exhibited a second primary cancer as a synchronous lesion in the thyroid gland, which is uncommon. All carcinomas, both in the tongue and thyroid gland, were removed surgically, and especially in the tongue, an anterolateral thigh free flap was performed successfully to replace the defect.
Spindle cell carcinoma (SpCC) is a rare type of squamous cell carcinoma (SCC) consisting of poorly differentiated elongated epithelial cells that show sarcoma-like proliferation1. SpCC has been called by various names, including pseudosarcoma, carcinosarcoma, pleomorphic carcinoma, and spindle cell squamous carcinoma. These designations represent its complex characteristics; it is a variation of SCC and has sarcomatoid cell composition2.
SpCC is a rare malignancy of the head and neck region found in the larynx, gingiva, tongue, hypopharynx, and nasal cavity in order of decreasing frequency. Most SpCC cases in the head and neck manifest in male patients between the sixth and eighth decades of life and in patients who have habits such as smoking and alcohol abuse or medical history of radiation exposure3.
The spindle cell components in SpCC are either a modified growth pattern of squamous cells associated with a non-neoplastic mesenchymal reaction or a malignant compound composed of epithelial and mesenchymal neoplasms. One histopathological feature used to identify SpCC is poorly differentiated squamous carcinoma including simultaneous sarcomatoid transformation demonstrated by the presence of malignant fusiform cell proliferation4. A widely accepted theory suggests that fusiform cells undergo a monoclonal epithelial neoplasia, which is influenced by related squamous carcinoma cells2.
The terms "second primary tumor" (SPT) and "double primary tumor" were proposed to explain a second tumor that develops independent of the first tumor. SPTs can appear as two types, synchronous or metachronous. Synchronous SPTs are defined as tumors that occur simultaneously or within 6 months of each other; metachronous SPTs occur more than 6 months apart based on the time of diagnosis of the first tumor5. The criteria to diagnose SPT from Warren and Gates6 are as follows: 1) all of the tumors have to be histologically malignant; 2) all tumors have to be distinct masses, separated by normal tissue (at least 2 cm); and 3) the possibility that the tumors could be metastases from existing primary tumor sites has to be histologically excluded.
The reported incidence of these SPTs varies, depending in part on the length of patient follow-up. Among head and neck cancer patients, an average of 15% are affected7, with reported incidences ranging from 2% to 16%5.
An evaluation of the original primary cancer and a search for a second primary malignancy should include not only detailed medical history taking and careful investigation, but also a radiological study of anatomic sites using computed tomography (CT) and magnetic resonance imaging (MRI), as well as panendoscopy. The efficacy of 18F-labeled fluorodeoxyglucose (FDG) positron emission tomography (PET) or fusion images of PET with CT (PET/CT) has also been reported8.
The patient introduced in this report had SpCC in the oral cavity (tongue) and a simultaneous malignant tumor at the thyroid gland. Together, these tumors were defined as synchronous cancers.
A 54-year-old male patient was taken to the dental hospital with the chief complaint of tongue pain that had arisen one month prior. The patient's social history included smoking 1 pack of cigarettes per day and alcohol consumption. He had no previous underlying disease or diagnosis of malignancy.
Upon clinical examination, a firm mass was located on the right ventral side of the tongue; movement of the tongue was difficult due to the mass. Mouth opening was normal. Incisional biopsy of the tongue lesion was conducted, and the lesion was diagnosed as SpCC. Imaging studies including neck CT and PET/CT showed the existence of a neoplastic lesion on the tongue and no evidence of adjacent bony invasion. Both neck sites had metastatic lesions, and a suspicious neoplastic aspect on the left side of thyroid gland was also visible.(Fig. 1
2
3) The tongue was classified clinically as T4aN2cMX (according to the classification of the American Joint Committee on Cancer [AJCC]; Tables 1
2
3)9, and a histopathologic examination of the thyroid gland was performed.
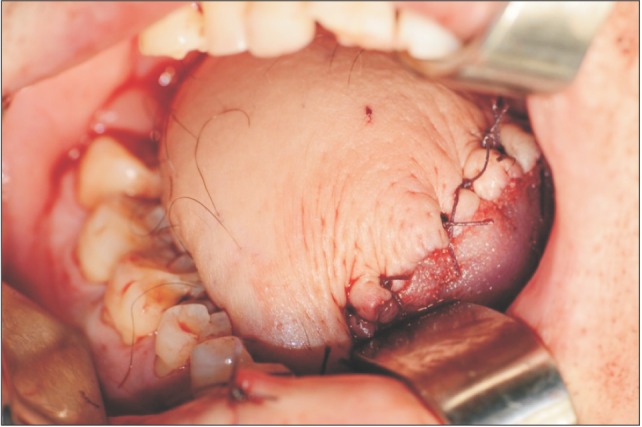
A partial glossectomy without jaw resection, bilateral lymphatic neck dissection, and anterolateral thigh free flap for tongue reconstruction were performed.(Fig. 4, 5) The patient was hospitalized for 3 weeks following the surgery. After the first surgery, the result of histopathologic examination of thyroid gland demonstrated that the lesion was malignant and not metastatic, but rather a separate tumor. The patient was referred to the department of otolaryngology for surgery on the thyroid lesion.
Histopathologic findings revealed the coexistence of keratinized pearls, which are characteristic changes of common squamous carcinoma cells, with sarcomatoid squamous cells that are relatively poorly differentiated, alongside fusiform-shaped cells.(Fig. 6) Another finding from the thyroid specimen was "papillary type" thyroid carcinoma, one of the most common malignant tumors of the thyroid gland. Fig. 7 shows that a significant portion of the thyroid gland cells had experienced papillary change, whereas a small portion of normal thyroid gland cells are present in the right upper corner.
After all surgeries were completed, the patient was referred to the department of hemato-oncology for adjunctive radiation therapy (computer-controlled radiation therapy) to eradicate the remaining tumor cells and undetected small lesions. During a 7-month follow-up period including 4 months after the surgery and 3 months admission time for adjunctive radiation therapy, we have found no significant changes at the surgical sites.
Written informed consent was obtained from the patient for the publication of this report and any accompanying images.
It has been reported that a phenotypic change occurs in the epithelial cells of SpCC that results in mesenchymal differentiation with production of a mesenchymal matrix component10. Also, in the inflammatory state, these cells develop a spindle-shaped morphology to migrate more easily. These facts imply that the spindle cell pattern may be related to the invasiveness and metastasis of disease1.
SpCC complicates histopathological diagnosis because it can present with remarkable morphological and immunohistochemical similarities to other benign and malignant tumors composed of spindle cells. An accurate diagnosis is important because of key differences in clinical management and prognosis11. In our case, sarcomatoid changes were seen upon histopathological evaluation using an optical microscope after H&E staining, so additional immunohistochemical steps were not necessary. If the diagnosis of SpCC is not clear by optical microscopic examination, further evaluation can be performed. Immunohistochemical study including analysis of the vimentin and cytokeratin coexpression in cells that comprise the tumor is essential for an accurate diagnosis; it is also an important parameter that influences selection of accurate therapeutic options1213. Radiological study should be taken into consideration to prevent an incorrect diagnosis of SpCC, such as mucosal spindle cell melanoma, leiomyosarcoma, myoepithelial carcinoma, or osteosarcoma extending to the mucosa10.
SpCC has high rates of recurrence and metastasis with disease progression. In this study, we found that metastasis to both neck lymph nodes existed according to PET/CT imaging. Results from the radiologic study corresponded with those from the histopathologic study completed on specimens collected during the neck dissection surgery.
Surgical resection is the most preferable and definitive treatment choice in many oral cancers because it is simple and shows relatively good results in terms of cure and postoperative function. The goals are to obliterate the tumor, preserve or restore form and function of anatomical landmarks, minimize the treatment process, and finally prevent subsequent primary cancers13.
Along with surgical resection, radiation therapy and chemotherapy can be used as adjunctive therapy1112. In general, prognosis after radiation therapy alone to treat SpCC has not been satisfactory14. Radiation therapy may be helpful in improving local control in patients with positive surgical margins. To improve local control, excision with a much wider safety margin (>2 cm) for SpCC would be helpful. To identify early recurrence, close postoperative follow-up is necessary. If recurrence occurs, a salvage operation is indicated and is helpful1.
The survival rate of SpCC is still controversial. One study of 59 oral SpCC cases by Ellis and Corio15 showed a 36% survival rate. Su et al.1 reported that the 2-year survival rate among oral SpCC patients was 27.5%. Su et al.1 also claimed that the survival rate of early stage patients (AJCC classification stages I and II) was relatively higher than that of late stage patients (stages III and IV). Leventon and Evans16 found that the survival of SpCC patients was dependent on whether tumors invaded superficially or deeply. They reported that deeper invasions were associated with lower survival rates.
PET and PET/CT are useful mechanisms for synchronous cancer detection. Haerle et al.8 proposed that FDG-PET is a more valuable modality to detect synchronous second primary malignancies than panendoscopy. In this case, double primary cancers of the tongue and thyroid gland including neck lymph node metastasis were successfully detected by PET/CT and MRI.(Fig. 1
2
3) However, superficial lesions of the head and neck region, at T1 or Tis (Table 1) level invasion, were identified as false-negatives on PET and PET/CT imaging. Thus, panendoscopy is mandatory for detection of superficial lesions in the upper gastrointestinal tract and head and neck region5.
According to various studies on multiple primary oral SCCs, 5-year survival in synchronous oral SCC patients is approximately 22% to 42%, in comparison with 54% to 76% for those with only a single primary malignancy. There have also been some studies comparing the prognosis of patients with synchronous cancers and metachronous cancers1718. Those studies have reported that the prognosis of patients with synchronous cancers is significantly lower than that of patients with metachronous cancers, and that second primary malignancies in the esophagus or lung contribute to a poorer outcome relative to other types. This suggests that the decline in survival rates in patients with SPTs is due to high morbidity requiring multiple surgeries, lack of well-established treatment in managing multifocal oral cancers, restricted therapeutic options, and relevant habits of the patient1920.
We think it is worthwhile to report this case because SpCC arising in the oral cavity combined with synchronous malignancy in other organs is a rare occurrence. As previously mentioned, oral SpCC and primary cancer of an additional site are associated with poor prognosis and low survival rate. At this time, we are now focused on close periodic follow-up and proper adjunctive therapy for the present patient.
References
1. Su HH, Chu ST, Hou YY, Chang KP, Chen CJ. Spindle cell carcinoma of the oral cavity and oropharynx: factors affecting outcome. J Chin Med Assoc. 2006; 69:478–483. PMID: 17098672.

2. Reyes M, Pennacchiotti G, Valdes F, Montes R, Veloso M, Matamala MA, et al. Sarcomatoid (spindle cell) carcinoma of tongue: a report of two cases. Case Rep Dent. 2015; 2015:780856. PMID: 25785207.

3. Lewis JE, Olsen KD, Sebo TJ. Spindle cell carcinoma of the larynx: review of 26 cases including DNA content and immunohistochemistry. Hum Pathol. 1997; 28:664–673. PMID: 9191000.

4. Wenig BM. Squamous cell carcinoma of the upper aerodigestive tract: precursors and problematic variants. Mod Pathol. 2002; 15:229–254. PMID: 11904340.

5. Yabuki K, Kubota A, Horiuchi C, Taguchi T, Nishimura G, Inamori M. Limitations of PET and PET/CT in detecting upper gastrointestinal synchronous cancer in patients with head and neck carcinoma. Eur Arch Otorhinolaryngol. 2013; 270:727–733. PMID: 22722946.

6. Warren S, Gates O. Multiple primary malignant tumors: a survey of the literature and statistical study. Am J Cancer. 1932; 16:1358–1414.
7. Cooper JS, Pajak TF, Rubin P, Tupchong L, Brady LW, Leibel SA, et al. Second malignancies in patients who have head and neck cancer: incidence, effect on survival and implications based on the RTOG experience. Int J Radiat Oncol Biol Phys. 1989; 17:449–456. PMID: 2674073.

8. Haerle SK, Strobel K, Hany TF, Sidler D, Stoeckli SJ. (18)F-FDG-PET/CT versus panendoscopy for the detection of synchronous second primary tumors in patients with head and neck squamous cell carcinoma. Head Neck. 2010; 32:319–325. PMID: 19626642.

9. Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al. American Joint Committee on Cancer (AJCC) cancer staging manual. 6th ed. New York: Springer Science and Business Media;2003.
10. Viswanathan S, Rahman K, Pallavi S, Sachin J, Patil A, Chaturvedi P, et al. Sarcomatoid (spindle cell) carcinoma of the head and neck mucosal region: a clinicopathologic review of 103 cases from a tertiary referral cancer centre. Head Neck Pathol. 2010; 4:265–275. PMID: 20730609.

11. Thompson LD, Wieneke JA, Miettinen M, Heffner DK. Spindle cell (sarcomatoid) carcinomas of the larynx: a clinicopathologic study of 187 cases. Am J Surg Pathol. 2002; 26:153–170. PMID: 11812937.
12. Ellis GL, Langloss JM, Heffner DK, Hyams VJ. Spindle-cell carcinoma of the aerodigestive tract. An immunohistochemical analysis of 21 cases. Am J Surg Pathol. 1987; 11:335–342. PMID: 2437812.
13. Shah JP, Gil Z. Current concepts in management of oral cancer: surgery. Oral Oncol. 2009; 45:394–401. PMID: 18674952.
14. Lambert PR, Ward PH, Berci G. Pseudosarcoma of the larynx: a comprehensive analysis. Arch Otolaryngol. 1980; 106:700–708. PMID: 7425942.

15. Ellis GL, Corio RL. Spindle cell carcinoma of the oral cavity. A clinicopathologic assessment of fifty-nine cases. Oral Surg Oral Med Oral Pathol. 1980; 50:523–533. PMID: 6935609.
16. Leventon GS, Evans HL. Sarcomatoid squamous cell carcinoma of the mucous membranes of the head and neck: a clinicopathologic study of 20 cases. Cancer. 1981; 48:994–1003. PMID: 7272943.

17. Dequanter D, Shahla M, Lardinois I, Gilbert O, Hanquet O, Tragas G, et al. Second primary lung malignancy in head and neck cancer patients. Eur Ann Otorhinolaryngol Head Neck Dis. 2011; 128:11–13. PMID: 20850406.

18. Rennemo E, Zätterström U, Boysen M. Impact of second primary tumors on survival in head and neck cancer: an analysis of 2,063 cases. Laryngoscope. 2008; 118:1350–1356. PMID: 18496157.
19. Liao CT, Kang CJ, Chang JT, Wang HM, Ng SH, Hsueh C, et al. Survival of second and multiple primary tumors in patients with oral cavity squamous cell carcinoma in the betel quid chewing area. Oral Oncol. 2007; 43:811–819. PMID: 17174143.

20. Shiga K, Tateda M, Katagiri K, Nakanome A, Ogawa T, Asada Y, et al. Distinct features of second primary malignancies in head and neck cancer patients in Japan. Tohoku J Exp Med. 2011; 225:5–12. PMID: 21817851.

Fig. 1
Primary tongue cancer presenting as a lobulated hypermetabolic lesion (maximum standardized uptake value [SUVmax] 8.7) in and around the anterior tongue and a focal mild hypermetabolic lesion (SUVmax 2.9) at the right cervical level I showed right neck lymph node metastasis in preoperative positron emission tomography and computed tomography (PET/CT).
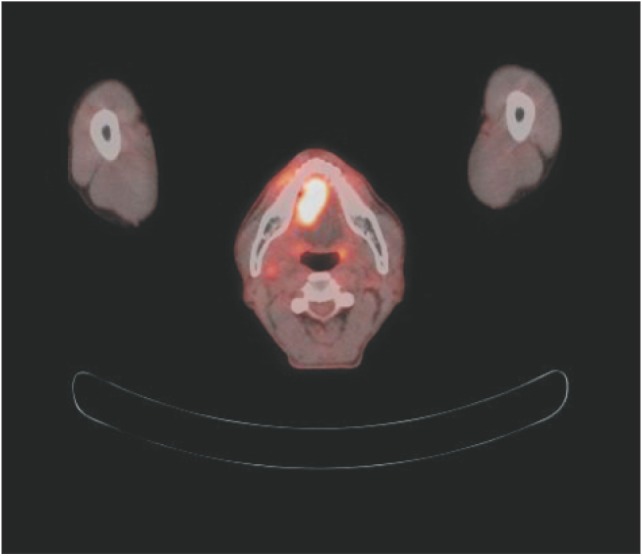
Fig. 2
A focal hypermetabolic lesion (maximum standardized uptake value 8.4) at the left cervical level III assumed to be a metastatic lymphadenopathy in preoperative positron emission tomography and computed tomography (PET/CT).

Fig. 3
A focal hypermetabolic lesion (maximum standardized uptake value 4.4) with calcification in the left thyroid gland suspected of being synchronous primary thyroid carcinoma in preoperative positron emission tomography and computed tomography (PET/CT).
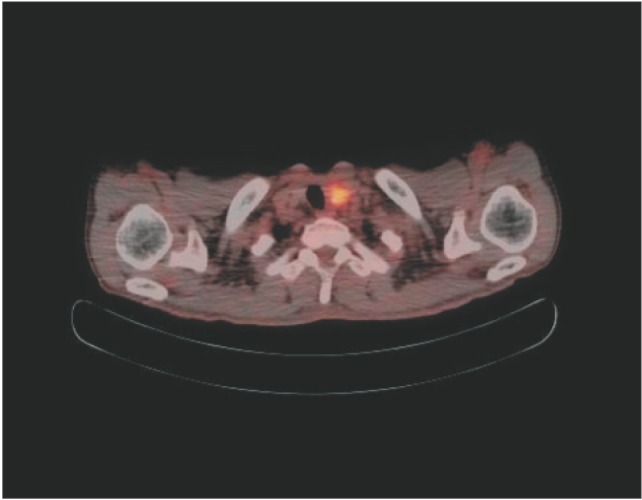
Fig. 4
Intra-operative photograph right after a subtotal glossectomy and modified radical neck dissection with visor flap.

Fig. 6
Presence of both poorly-differentiated sarcomatoid changes of cells and keratinized pearls (arrows) which are characteristic features of squamous cell carcinoma (H&E staining, ×100).
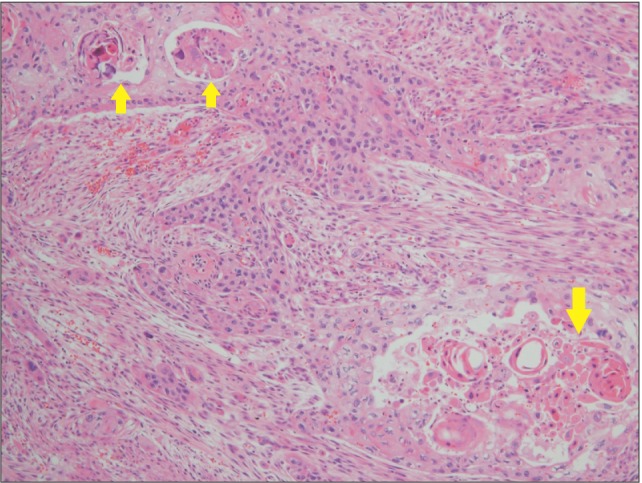
Fig. 7
Typical cells of papillary carcinoma are predominantly seen in this specimen; some normal thyroid gland cells are noted in the right upper corner (H&E staining, ×100).
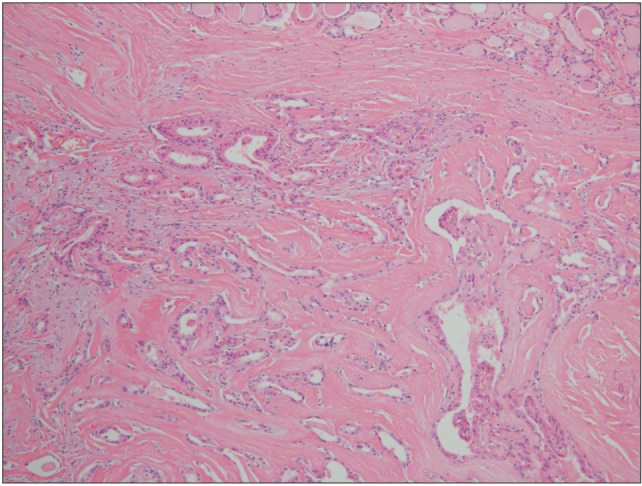
Table 1
Primary tumor (T) classification of TNM in oral cavity cancer
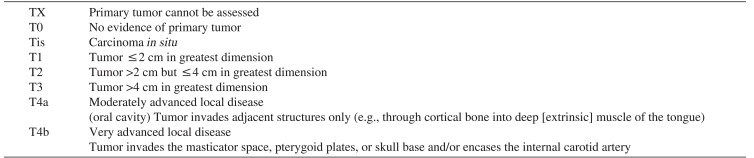
Cited from American Joint Committee on Cancer (AJCC) cancer staging manual. 6th ed. (New York: Springer Science and Business Media; 2003)9.
Table 2
Regional lymph nodes (N) classification of TNM in oral cavity cancer
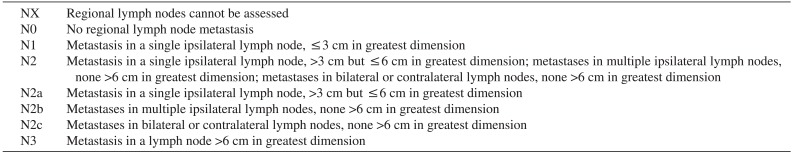
Cited from American Joint Committee on Cancer (AJCC) cancer staging manual. 6th ed. (New York: Springer Science and Business Media; 2003)9.
Table 3
Distant metastasis (M) classification of TNM in oral cavity cancer

| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
Cited from American Joint Committee on Cancer (AJCC) cancer staging manual. 6th ed. (New York: Springer Science and Business Media; 2003)9.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download